ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 10
Elevated serum CXCL13 level in patients with bronchial asthma relates to clinical presentations
Conghui Wang and Rongyu Liu*
Department of Pulmonology, Anhui Geriatric Institute, the First Affiliated Hospital of Anhui Medical University, PR China
- *Corresponding Author:
- Rongyu Liu
Department of Pulmonology
Anhui Geriatric Institute
The First Affiliated Hospital of Anhui Medical University
PR China
E-mail: rongyuliu@163.com
Accepted date: February 28, 2017
Background: Asthma is an allergic airway inflammatory disease in which B cell activation plays a pivotal role. CXCL13 is a family member of CXC chemokines involved in B cell differentiation and activation. The purpose of this research is to explore the correlation between serum CXCL13 levels and the severity of the disease.
Methods: A total of 31 patients with acute bronchial asthma, 29 patients with chronic persistent asthma, and 21 non-inflammatory disease healthy controls were enrolled. Quantification of serum CXCL13, IgE, peripheral blood Eosinophil (Eos) count, and pulmonary function were performed.
Results: Both the acute bronchial asthma and the chronic persistent groups displayed higher levels of CXCL13, and serum CXCL13 levels in acute asthma patients were higher than those suffering chronic persistent disease. Serum CXCL13 levels in acute bronchial asthma patients were positively correlated with IgE levels and the frequency of Eos, and negatively correlated with FVC%, FEV1% and FEV1/ FVC.
Conclusions: CXCL13 may be an important biomarker of acute exacerbation of bronchial asthma, which concentration might reflect the severity of the disease.
Keywords
CXCL13, Asthma, IgE, Ventilatory functions, Eosinophils
Introduction
Bronchial asthma is an allergic airway inflammatory disease that involves a variety of cells, such as eosinophils, mast cells, T lymphocytes, neutrophils, smooth muscle cells, airway epithelial cells, etc., and cellular components. It is reported that B cells were required for airway inflammation, Th2 cytokine production, and AHR [1]. Multiple studies have found that B cells play an important role in the pathogenesis of bronchial asthma [2-4]. B cells are generally considered to positively regulate immune responses by producing antigen-specific antibodies [5,6]. B cells synthesize immunoglobulin Ig E following exposure of a dendritic cell to a foreign antigen under the control of CD4+ T-helper lymphocytes [7,8].
CXCL13, also known as the BLC/BCA-1 (B-lymphocyte chemoattractant/B-cell attracting chemokine-1), is a family member of the CXC chemokines. CXCL13 and its receptor play an important role in targeting B cells and the formation of B lymphocyte zone in lymphoid tissues [9]. Interestingly, a high level of expression of CXCL13 mRNA was found in Bronchoalveolar Lavage Fluid (BALF) of bronchial asthma patients [10]. However, there is no evidence on the incidence of CXCL13 in patients with bronchial asthma and lung function currently.
The purpose of this study is to determine the role of CXCL13 in the pathogenesis of bronchial asthma. We performed a correlative analysis between the levels of CXCL13 expressed by circulating B lymphocytes of patients suffering from asthma at various degree of severity, and the levels of IgE, the frequency of Eos, and lung function.
Patients and Methods
Patients
We selected 31 patients diagnosed with typical asthma in acute phase, and 29 patients in chronic stage from the First Affiliated Hospital of Anhui Medical University. Demographics and patient clinical features were shown in Table 1. The main inclusion criteria were a diagnosis of asthma according to the Global Initiative for Asthma 2012 [11]. In addition, 21 healthy subjects were recruited as the control group. There was no significant difference in age and gender among the patients and controls. None of the patients used glucocorticoid during the two weeks prior to testing, nor did they use theophylline or receptor agonists in the 72 h before measurements. Patients were all informed and agreed. The study was approved by Hospital Medical Ethics Committee and all participants provided written informed consent prior to study.
| Acute paroxysmal asthma (n=31) | Chronic persistent asthma (n=29) | Controls (n=21) | |
|---|---|---|---|
| Gender (Female/male) | 16/15 | 15/14 | 12-Sep |
| Age (years) | 37.45 ± 11.57 | 35.68 ± 12.04 | 32.14 ± 9.95 |
| Course of disease (years) | 9.56 ± 8.99 | 3.93 ± 5.26 | NA |
| FVC | 2.84 ± 1.05 | 3.76 ± 0.91 | 4.13 ± 0.70 |
| FVC% | 65.98 ± 14.58 | 93.88 ± 13.17 | 104.66 ± 11.53 |
| FEV1% | 52.25 ± 13.55 | 96.55 ± 14.67 | 112.23 ± 10.82 |
| FEV1/FVC | 0.77 ± 0.15 | 0.87 ± 0.10 | 0.91 ± 0.06 |
| MMF% | 65.41 ± 40.08 | 94.96 ± 44.69 | 133.50 ± 29.09 |
| Eosinophil’s (%) | 8.29% ± 5.41 | 3.93% ± 2.75 | 4.68% ± 2.39 |
| FVC: Forced Vital Capacity; FEV1: Forced Expiratory Volume in one second; MMF: Maximal Mid expiratory Flow | |||
Table 1: Demographic characteristics of AS patients and healthy controls.
Methods
Determination of serum CXCL13 levels in asthma patients by ELISA: Peripheral venous blood samples were taken from all subjects. 1000 g of blood was centrifuged for 10 min; serum was frozen at -80°C until testing. CXCL13 and IgE serum levels were quantified using ELISA (CXCL13, R&D Systems, USA; IgE, Bender MedSystems, Austria) according to the manufacturer’s instructions.
Count of Eosinophils in peripheral blood: Venous blood (2 ml) was taken from each subject, and the number of Eos was counted using the Sysmex XE-2100 hematology analyser.
Spirometry: Japan HI-701 spirometer was used for all patients. Three times of FVC (Forced Vital Capacity), FEV1 (Forced Expiratory Volume in one second) and PEF (Peak Expiratory Flow) measurements with stable results were recorded, and the best values were chosen for the study.
Statistical methods: Data were presented as mean ± standard deviation. Differences in levels of CXCL13 and IgE between different subgroups were analysed using Mann-Whitney U test. Correlations between CXCL13, IgE, and eosinophil percentage were analysed using Spearman's rank test. P value<0.05 was considered statistically significant. All statistical analyses were performed using SPSS 16.0 (SPSS Inc. Chicago, IL, USA) for windows
Results
CXCL13 and serum IgE concentrations in serum of each group
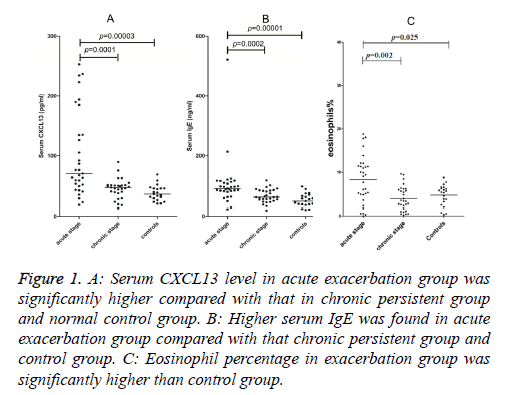
Serum CXCL13 concentrations in the acute exacerbated asthma was 100.67 ± 70.95 pg/ml, 45.35 ± 16.28 pg/ml in chronic persistent and 37.99 ± 12.77 pg/ml in control groups (Figure 1A). Serum CXCL13 level in acute asthma was significantly higher than chronic asthma (p=0.0001) and healthy controls (p=0.00003), whereas there was no significant difference between the chronic persistent and control groups.
Figure 1: A: Serum CXCL13 level in acute exacerbation group was significantly higher compared with that in chronic persistent group and normal control group. B: Higher serum IgE was found in acute exacerbation group compared with that chronic persistent group and control group. C: Eosinophil percentage in exacerbation group was significantly higher than control group.
Serum IgE concentrations in acute, chronic and healthy groups were 132.42 ± 94.19 ng/ml, 66.38 ± 22.28 ng/ml, and 57.71 ± 37.02 ng/ml, respectively (Figure 1B). However, higher levels were found in patients suffering from acute asthma than patients with chronic persistent disease (p=0.0002) or healthy subjects (p=0.00001). No significant difference was found between the chronic persistent and control groups.
The eosinophil percentage in the blood of each group
Percentages of Eos in the blood of patients with acute exacerbated, chronic persistent disease and the control group were 8.29% ± 5.41%, 3.93% ± 2.75%, 4.68% ± 2.39%, respectively (Figure 1C). The percentage of Eos in the acute exacerbated asthma group was significantly higher than the control group (p=0.025).
Correlations between the level of CXCL13 and IgE, eosinophil percentage or different ventilatory functions in the acute stage of bronchial asthma
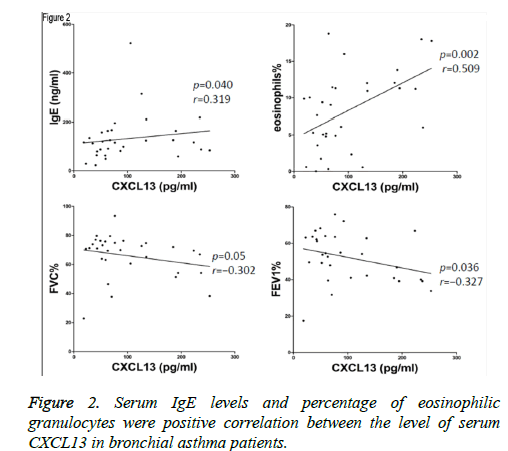
Levels of CXCL13 were positively correlated with concentration of IgE (p=0.040, r=0.319), and eosinophilia% (p=0.002, r=0.509) in acute exacerbated asthma (Figure 2). However, Levels of CXCL13 were negatively correlated with FVC% (p=0.05, r=-0.302), and FEV1% (p=0.036, r=-0.327). Furthermore, there was no significant correlation between the level of CXCL13 and FEV1/FVC (p=0.154, r=-0.190).
Discussion
In this study, we found that CXCL13 levels in the serum of patients presenting with acute exacerbated symptoms of bronchial asthma were significantly elevated, which was consistent with increased IgE levels and frequency of eosinophilia. However, CXCL13 was negatively correlated with FVC%, FEV1% and FEV1/FVC.
CXCLl3 is one of the CXC chemokine family factors known as B Cell-Attracting chemokine 1 (BCA-1) or B-1ymphocyte Chemoattractant (BLC). CXCL13, and its receptor, play an important role in B cell targeting, and the formation of the B cell region in lymphoid tissues. High expression of CXCL13 has been reported in various types of lung disease, such as chronic obstructive pulmonary disease [12-14], pulmonary embolism [15], idiopathic pulmonary fibrosis [16,17], lung cancer [18,19]. It has also been reported in other inflammatory diseases such as autoimmune sinusitis [20,21], rheumatoid arthritis [22], Sjogren's syndrome [23], multiple sclerosis [24], lupus kidney [25], optic neuritis [26], which further validated CXCL13 as a crucial factor in the development of chronic inflammatory diseases. Guzman et al. [9] have reported that the expression of CXCL13 is elevated in the lung of OVA-sensitized mice. Interestingly, neutralization of CXCL13 reduced airway inflammation in sensitized mice. This chemokine has been linked to the formation of bronchus-associated lymphoid tissues, B cells, follicular CXCR5+ and cell recruitment. Additionally, higher expression levels of CXCL13 have also been detected in BALF from patients with asthma than healthy subjects, which is in agreement with the results generated in this study. High levels of CXCL13 may promote humoral immunity and enhance an autoimmune inflammatory component in asthma patients. We also found that high levels of serum CXCL13 were consistent with elevated levels of IgE in patients with acute asthma. Thus, CXCL13 may reflect the inflammation level of bronchial asthma.
IgE is the fifth and the last discovered immunoglobulin, which is a key factor of asthma airway hyperreactivity induction. Epidemiological studies have shown that total levels of IgE in asthma patients were higher than in non-asthmatic subjects, especially children [27-29]. A recent survey from the European Community Respiratory Health showed that total IgE was related to atopic allergic new-onset asthma [30,31]. Marco et al. have studied 856 cases of asthma in Europe and found that high levels of IgE tend to correlate to moderate to severe asthma [32]. CXCL13 promotes B cell translocation to sites of inflammation to be further activated via CXCR5 receptor ligation on the B cell surface, which may in turn increase IgE synthesis. This hypothesis could explain the increase of CXCL13 and IgE in patients with asthma, as well as their positive correlation.
Eosinophils are a major effector component in bronchial asthma and play a key role in allergic inflammatory diseases. We reported that serum CXCL13 levels were positively correlated with frequency of eosinophilia. Previous studies have shown that high levels of eosinophil of asthma patients were related to decreased FEV1 [33,35]. Thus, the percentage of eosinophils was used as an indicator of airway inflammation. In the present study, the percentage of eosinophil was positively correlated with CXCL13, which is in agreement with our hypothesis that elevated CXCL13 may enhance inflammation in patients with acute bronchial asthma.
Moore et al. have reported that significant changes in lung function were observed in patient with occupational asthma as shown by decreased PEF ratio in the afternoon and evening in comparison to daytime [36]. Yamasaki et al. further pointed a relationship between sleep disorder in asthma patients and reduced lung function [37]. However, the correlation between lung function in patients with acute exacerbation, chronic persistent bronchial asthma or healthy subjects with serum CXCL13 levels has not been studied. In this study, we found that lung function, measured by FVC%, FEV1% and FEV1/ FVC, in patients presenting with acute exacerbation of bronchial asthma were lower than in patients suffering from chronic persistent asthma or than the healthy group, suggesting airflow limitation and a ventilatory function decline during the acute exacerbation of bronchial asthma. Serum CXCL13 level of asthma patients was negatively correlated to FVC%, FEV1%, suggesting that airway inflammation and airway limitation was progressively increased. However, the correlation of CXCL13 level and FEV1%/FVC was not statistically significant, which might be due to the relatively limited number of samples.
Conclusion
CXCL13 concentration seems to be related to the severity of acute bronchial asthma. However, due to the limited number of samples in this study, more work has to be performed to evaluate CXCL13 as a new target in the treatment of asthma.
Acknowledgement
None
Conflict of Interest
None
References
- Drake LY, Iijima K, Hara K, Kobayashi T, Kephart GM. B cells play key roles in th2-type airway immune responses in mice exposed to natural airborne allergens. PLoS One 2015; 10: 0121660.
- Moser M, Halwani R, Al-Kufaidy R, Pureza MA, BaHammam AS, Al-Jahdali H, Alnassar SA, Hamid Q, Al-Muhsen S. IL-17 enhances chemotaxis of primary human b cells during asthma. PLoS One 2014; 9: 114604.
- Liao HY, Tao L, Zhao J, Qin J, Zeng GC, Cai SW, Li Y, Zhang J, Chen HG. Clostridium butyricum in combination with specific immunotherapy converts antigen-specific B cells to regulatory B cells in asthmatic patients. Sci Rep 2016; 6: 20481.
- Yuan Sl, Zhong P, Fan XM, Huang CL, Wang WJ, Zhan XQ, He XP. B cell activating transcription factor regulates acute airway inflammation in asthmatic mice. Sichuan Da Xue Xue Bao Yi Xue Ban 2015; 46: 533-536.
- Lucas M, Jeelall Y, Kavanagh S, Bundell C, Hew M, Wood BA, Joske D, McLean-Tooke A. B-cell small lymphocytic lymphoma associated with extremely high total IgE and cutaneous vasculitis. J Allergy Clin Immunol Pract 2016; 4: 552-554.
- Zan H, Casali P. Epigenetics of peripheral b-cell differentiation and the antibody response. Front Immunol 2015; 6: 631.
- Endo Y, Hirahara K, Yagi R, Tumes DJ, Nakayama T. Pathogenic memory type Th2 cells in allergic inflammation. Trends Immunol 2014; 35: 69-78.
- Song GG, Lee YH. Pathway analysis of genome-wide association study on asthma. Hum Immunol 2013; 74: 256-260.
- Finch DK, Ettinger R, Karnell JL, Herbst R, Sleeman MA. Effects of CXCL13 inhibition on lymphoid follicles in models of autoimmune disease. Eur J Clin Invest 2013; 43: 501-509.
- Baay-Guzman GJ, Huerta-Yepez S, Vega MI, Aguilar-Leon D, Campillos M, Blake J, Benes V, Hernandez-Pando R, Teran LM. Role of CXCL13 in asthma: novel therapeutic target. Chest 2012; 141: 886-894.
- Global Initiative for Asthma. GINA report, Global Strategy for Asthma Management and Prevention 2012.
- Litsiou E, Semitekolou M, Galani IE, Morianos I, Tsoutsa A, Kara P, Rontogianni D, Bellenis I, Konstantinou M, Potaris K, Andreakos E, Sideras P, Zakynthinos S, Tsoumakidou M. CXCL13 Production in B cells via toll-like receptor/lymphotoxin receptor signaling is involved in lymphoid neogenesis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 1194-1202.
- Bracke KR, Verhamme FM, Seys LJ, Bantsimba-Malanda C, Cunoosamy DM, Herbst R, Hammad H, Lambrecht BN, Joos GF, Brusselle GG. Role of CXCL13 in cigarette smoke-induced lymphoid follicle formation and COPD. Am J Respir Crit Care Med 2013; 188: 343-355.
- Bracke KR, Verhamme FM, Seys LJ, Bantsimba-Malanda C, Cunoosamy DM, Herbst R, Hammad H, Lambrecht BN, Joos GF, Brusselle GG. Role of cxcl13 in cigarette smoke-induced lymphoid follicle formation and chronic obstructive pulmonary disease. Am J Resp Crit Care Med 2013; 188: 343-355.
- Lv W, Duan QL, Wang LM, Gong Z, Yang F, Song YL. Gene expression levels of cytokines in peripheral blood mononuclear cells from patients with pulmonary embolism. Mol Med Rep 2013; 7: 1245-1250.
- Vuga LJ, Tedrow JR, Pandit KV, Tan J, Kass DJ, Xue J, Chandra D, Leader JK, Gibson KF, Kaminski N, Sciurba FC, Duncan SR. C-X-C motif chemokine 13 (CXCL13) is a prognostic biomarker of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2014; 189: 966-974.
- DePianto DJ, Chandriani S, Abbas AR, Jia G, NDiaye EN, Caplazi P, Kauder SE, Biswas S, Karnik SK, Ha C, Modrusan Z, Matthay MA, Kukreja J, Collard HR, Egen JG, Wolters PJ, Arron JR. Heterogeneous gene expression signatures correspond to distinct lung pathologies and biomarkers of disease severity in idiopathic pulmonary fibrosis. Thorax 2015; 70: 48-56.
- Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 2013; 105: 1871-1880.
- Singh R, Gupta P, Kloecker GH, Singh S, Lillard JW. Expression and clinical significance of CXCR5/CXCL13 in human nonâ€small cell lung carcinoma. Int J Oncol 2014; 45: 2232-2240.
- Wu X, Ghimbovschi S, Aujla PK, Rose MC, Pena MT. Expression profiling of inflammatory mediators in pediatric sinus mucosa. Arch Otolaryngol Head Neck Surg 2009; 135: 65-72.
- Patadia M, Dixon J, Conley D, Chandra R, Peters A, Suh LA, Kato A, Carter R, Harris K, Grammer L, Kern R, Schleimer R. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am J Rhinol Allergy 2010; 24: 11-16.
- Jones JD, Hamilton BJ, Challener GJ, de Brum-Fernandes AJ, Cossette P, Liang P, Masetto A, Ménard HA, Carrier N, Boyle DL, Rosengren S, Boire G, Rigby WF. Serum C-X-C motif chemokine 13 is elevated in early and established rheumatoid arthritis and correlates with rheumatoid factor levels. Arthritis Res Ther 2014; 16: 103
- Kramer JM, Klimatcheva E, Rothstein TL. CXCL13 is elevated in Sjogrens syndrome in mice and humans and is implicated in disease pathogenesis. J Leuk Biol 2013; 94.
- Harris VK, Sadiq SA. Biomarkers of therapeutic response in multiple sclerosis: current status. Mol Diagn Ther 2014; 18: 605-617.
- Worthmann K, Gueler F, von Vietinghoff S, Davalos-Miblitz A, Wiehler F, Davidson A, Witte T, Haller H, Schiffer M, Falk CS, Schiffer L. Pathogenetic role of glomerular CXCL13 expression in lupus nephritis. Br Soc Immunol Clin Exp Immunol 2014; 178: 20-27.
- Hytonen J, Kortela E, Waris M, Puustinen J, Salo J, Oksi J. CXCL13 and neopterin concentrations in cerebrospinal fluid of patients with Lyme neuroborreliosis and other diseases that cause neuroinflammation. J Neuroinflamm 2014; 11: 103.
- Criqui MH, Seibles JA, Hamburger RN, Coughlin SS, Gabriel S. Epidemiology of immunoglobulin E levels in a defined population. Ann Allergy 1990; 64: 308-313.
- Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med 1989; 320: 271-277.
- Pinart M, Benet M, Annesi-Maesano I, von Berg A, Berdel D, Carlsen KC, Carlsen KH, Bindslev-Jensen C, Eller E, Fantini MP, Lenzi J, Gehring U, Heinrich J, Hohmann C, Just J, Keil T, Kerkhof M, Kogevinas M, Koletzko S, Koppelman GH, Kull I, Lau S, Melen E, Momas I, Porta D, Postma DS, Rancière F, Smit HA, Stein RT, Tischer CG, Torrent M, Wickman M, Wijga AH, Bousquet J7, Sunyer J, Basagana X, Guerra S, Garcia-Aymerich J, Anto JM. Comorbidity of eczema, rhinitis, and asthma in IgE-sensitised and non-IgE-sensitised children in MeDALL: a population-based cohort study. Lancet Respir Med 2014; 2: 131-140.
- Anto JM, Sunyer J, Asagana X, Garcia-Esteban R, Cerveri I, de Marco R, Heinrich J, Janson C, Jarvis D, Kogevinas M, Kuenzli N, Leynaert B, Svanes C, Wjst M, Gislason T, Burney P. Risk factors of new-onset asthma in adults: a population-based international cohort study. Allergy 2010; 65: 1021-1030.
- Siroux V, Oryszczyn MP, Paty E, Kauffmann F, Pison C, Vervloet D, Pin I. Relationships of allergic sensitization, total immunoglobulin E and blood eosinophils to asthma severity in children of the EGEA Study. Clin Exp Allergy 2003; 33: 746-751.
- de Marco R, Marcon A, Jarvis D, Accordini S, Almar E, Bugiani M, Carolei A, Cazzoletti L, Corsico A, Gislason D, Gulsvik A, Jogi R, Marinoni A, Martinez-Moratalla J, Pin I, Janson C, European Community Respiratory Health Survey Therapy Group. Prognostic factors of asthma severity: a 9-year international prospective cohort study. J Allergy Clin Immunol 2006; 117: 1249-1256.
- Ren YF, Li H, Xing XH, Guan HS, Zhang BA. Preliminary study on pathogenesis of bronchial asthma in children. Pediatr Res 2015; 77: 506-510.
- Ulrik CS, Backer V, Dirksen A. A 10 year follow up of 180 adults with bronchial asthma: factors important for the decline in lung function. Thorax 1992; 47: 14-18.
- Broekema M, Volbeda F, Timens W, Dijkstra A, Lee NA, Lee JJ, Lodewijk ME, Postma DS, Hylkema MN, Ten Hacken NH. Airway eosinophilia in remission and progression of asthma: accumulation with a fast decline of FEV1. Respir Med 2010; 104: 1254-1262.
- Moore VC, Jaakkola MS, Burge CB, Pantin CF, Robertson AS. Shift work effects on serial PEF measurements for occupational asthma. Occup Med (Lond) 2012; 62: 525-532.
- Akira Y, Kawasaki Y, Takeda K, Harada T, Fukushima T, Takata M, Hashimoto K, Watanabe M, Kurai J, Nishimura K, Shimizu E. The relationships among sleep efficiency, pulmonary functions, and quality of life in patients with asthma. Int J Gen Med 2014; 7 505-512.