ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2012) Volume 23, Issue 3
Evaluation of procedures for quantification of CD34+haematopoietic stem cells and viability studies in umbilical cord blood derived mononuclear cells
1Department of Transfusion Medicine and Blood Bank Chhattrapati Shahuji Maharaj Medical University ( Earlier King Georg’s Medical University), Lucknow Uttar Pradesh, India
2Department of Pathology Chhattrapati Shahuji Maharaj Medical University ( Earlier King Georg’s Medical University), Lucknow Uttar Pradesh, India
3Department of Obstetrics and Gynecology, Chhattrapati Shahuji Maharaj Medical University ( Earlier King Georg’s Medical University), Lucknow Uttar Pradesh, India
- *Corresponding Author:
- Tulika Chandra
Department of Transfusion Medicine and Blood Bank Chhatrapati Shahuji Maharaj Medical University
(Earlier King Georg’s Medical University) Lucknow 226003, Uttar Pradesh India
Mobile: +91-9415755536
E-Mail: tulikachandra@rediffmail.com
Accepted date: February 22 2012
Umbilical cord blood (UCB) transplantation is being used as an alternative source of haematopoietic stem cells for bone marrow reconstitution. Umbilical cord blood (UCB) transplantation is being used as an alternative source of haematopoietic stem cells for bone marrow reconstitution. Separation and processing of UCB samples in large numbers for storage in cord blood banks ideally needs to be partially automated. This study examines the loss of CD34+cells concentration and viability of mononuclear cells from baseline verses one month, baseline verses six month and one month verses six month as well as standardization of procedure for cryopresevation of cord blood derived haematopoietic stem cells. A total of 500 umbilical cord blood units were collected. Samples were analyzed for CD34+ cells concentration and viability of mononuclear cells at baseline, one month and six month. In present study we found that the mean CD34+cell loss from base line to one month was found to be 0.24?, baseline to six month was 0.78? and one month to six month was 0.54?. The mean viability of mononuclear cell loss from base line to one month was found to be 8.26?, baseline to six month was 74.20? and one month to six month was8.99?. Our data indicate that CD34+ cells and viability of mononuclear cells in UCB were maintained after cryopreservation of six month period in -800C. This amount of CD34+ cells were within the range used for successful engraftment in both related and unrelated cord blood transplantation.
Keywords
Umbilical cord blood, CD34+ cells, mononuclear cells, Viability, Cryopreservation
Introduction
Umbilical cord blood contains hematopoieticstem/ progenitor cells that have proven useful clinically to reconstitute the hematopoietic system in children and some adults [1].
Work that was begun in the early 1980s revealed that cord blood (ie, the leftover blood in the umbilical cord and placenta after the birth of a child) was comparable to bone marrow in terms of its utility in stem cell transplantation [2]. Cord blood offers a number of advantages over the bone marrow including a lower incidence of Graft verses host disease and less strict HLA-matching requirements, which could increase its availability to transplant patients [3]. During the past 10 years, clinical use of cord blood (with more than 8,000 transplants worldwide) has shown that it is a suitable alternative to bone marrow [4]. In addition to its use as a substitute for bone marrow, cord blood has recently been used in a variety of regenerative medicine applications. Work done by Harris and colleagues (2007) has shown that cord blood contains a mixture of pluripotent stem cells capable of giving rise to cells derived from the endodermal, mesodermal, and ectodermal lineages [5]. Thus, cord blood appears to be a practical substitute for embryonic stem cells and readily available for use in tissue engineering and regenerative medicine.
The cryopreservation process is of importance for all types of stem cell collection, but it is perhaps particularly critical for umbilical cord blood (UCB). The actual transplant is harvested at the time of birth and used at a later point in time for often an indeterminate recipient [6]. Cryopreservation of cord blood derived haematopoietic stem cells is critical for UCB banking, transplantation as well as for research applications by providing readily available specimens. Separation and processing of UCB samples in large number of storage in cord blood banks ideally needs to be partially automated to allow large numbers of samples to be processed efficiently. A closed system reduces the risk of bacterial contamination after collection. The processing method must allow for the adequate recovery of nucleated cells and progenitors to enable engraftment. Early attempts at separating cord blood by density gradient techniques led to loss of mononuclear cells which suggests that cord blood should be stored unseparated. However, volume reduction is essential for cord blood banks to be economical and efficient. Several different methods have been employed to reduce the volume prior to cryopreservation without loss of progenitor cells such as density gradient separation [7], sedimentation of red cells by gelatin [8], rouleaux formation induced by hydroxyethyl starch (HES) and centrifugation [9], and differential centrifugation with expression of RBC and plasma [10].
The present study was carried out to assess loss of CD34+cells concentration and viability of mononuclear cells from baseline verses one month, baseline verses six month and one month verses six month as well as standardization of procedure for cryopresevation of cord blood derived haematopoietic stem cells.
Materials and Methods
A total of 480 were obtained from both vaginal and caesarian deliveries from the Department of Obstetrics and Gynecology. Processing of 480 samples of UCB was done in the Department of Transfusion Medicine, Chattrapati Shahuji Maharaj Medical University, Lucknow. Written informed consent was obtained from mother.
The study has been approved by Institutional Ethical Committee of Chattrapati Shahu Ji Maharaj Medical University, Lucknow.
Inclusion Criteria
All healthy full term females with no history hepatitis, infectious disease, diabetes mellitus, severe hypertension, abortion or bad obstetrics. Infants delivered at term (31- 41 weeks) were included.
Exclusion Criteria
All females with APH, eclampsia and high risk cases were excluded from the study.
Cord blood donor infants
Birth weight, baby sex, mode of delivery and gestational age of the baby were also recorded. After collection, the cord blood was sent in the transport boxes to the department of Transfusion Medicine, Chattrapati Shahuji Maharaj Medical University, Lucknow, India.
A) Collection of UCB
Cord blood was collected from 370 (77.08%) normal vaginal and 110 (22.9%) cesarean deliveries after the completion of delivery before placenta expulsion in CPDA triple blood bag containing 69 ml anticoagulant citrate, phosphate, dextrose and adenine. 20 ml anticoagulant was removed before collection of umbilical cord blood. After the delivery of the baby the cord was clamped and wiped with alcohol or betadine to ensure sterility of the collection. A needle was inserted into the umbilical vein above the clamp. The blood was drained via gravity into the sterile collection bag, containing Citrate Phosphate Dextrose Adenine (CPDA) as an anticoagulant. Efforts were made to obtain maximal volumes from each collection. The umbilical cord blood units were stored at 4°C and processed within 24 hours. Samples of 3 ml per unit were taken at this stage for nucleated cell (NC) count.
B) Processing of UCB
Processing was done within 24 hours of cord blood collection. The CD34+ cell concentration, total nucleated cells count and viability assay were done on all the samples. Transmissible disease testing for Human Immunodeficiency Virus (HIV-1and 2), Hepatitis B Virus (HBV), Hepatitis C Virus (HCV) and syphilis was also performed. In the processing of samples, which included determination of cord blood volumes. Determination of initial level of total nucleated cells (TNC) and mononuclear cells (MNC) before centrifugation was done. The UCB product was mixed with HES containing solution (6% HES in 0.9 NaCl) in a 5: 1 ratio and centrifuged in a Cryofuge 6000i (Heraeus-Kendro, Hanau, Germany) at 1200 x g for 10 min. The WBC-rich plasma was expressed in a separate bag and again centrifuged at 2500 x g for 10 min. The WBC poor plasma was expressed and discarded. The remaining suspension of mononuclear cells was left whose counts were recorded. The complete process was performed in a closed system with the use of a sterile connecting device (Terumo TSCD, SC-201 AH, Leuven, Belgium).
C) Assessment of Total nucleated count, CD34+ and viability test
The samples were analyzed for CD34+ cells concentration and viability of mononuclear cell on baseline period, one month and six month. The total nucleated cells count was done before RBC depletion by automated cell counter (Sysmex KX-21, Japan). CD34+ cells concentration was done by flowcytometery and viability of mononuclear cells were done by trypan blue dye exclusion test.
D) Cryopreservation
Dimethyl sulfoxide (DMSO) (Merck Limited, Mumbai) was used at a final concentration of 10% (vol/vol). The required volume of sterile, chilled DMSO solution was added to the blood bag over the course of 15 min by using a syringe pump and an orbital mixer to assure smooth but vigorous mixing. In these experiments, UCB processed units were mixed with either 20% DMSO in saline or 50% DMSO in 5% (wt/vol) Dextran 40 (Mr 35,000- 50,000) (USB Corporation Cleveland, OH,USA). Final volumes of UCB units with DMSO was a uniform 25 ml. Cryoprotectant UCB units were kept cold with wet ice throughout the addition. When the concentration of DMSO reached 10%, cell suspensions were transferred to -20°C for 5-10 hours. Subsequently it was transferred to - 40°C for 4 hours and then stored at -80°C for six month.
E) Thawing and Washing
Umbilical cord blood processing method was done as previously described by Rubinstein and colleagues (New York Blood Center) [13]. UCB units were thawed and washed. Briefly, umbilical cord blood units were taken out of the -80°C and immersed in a 37°C water bath. After inspection, 10 % dextran 40 was slowly added followed by 5 percent human albumin and the bag was left to equilibrate for 5 minutes. The product was transferred to a first transfer bag and centrifuged at 400 x ıg for 15 minutes at 4°C. Cell pellet were obtained in first transfer bag and approximately three-fourths of the wash supernatant was expressed and transferred in a second transfer bag. The wash supernatant in the second transfer was again centrifuged at 800 xı g for 15 minutes at 4°C. Three fourth of the wash supernatant was expressed. The residual cell pellets obtained in the second transfer bag were combined in first transfer bag through sterile tube welder. The combined cell pellets were resuspended in 10 percent dextran and 5 percent human albumin.
Statistical Analysis
Data collected was entered in Microsoft excel and checked for any inconsistency. The mean and standard deviation were calculated at baseline, one month and six months for CD34+ cells concentration and viability of mononuclear cells count. The repeated measures of analysis (ANOVA) was used to compare loss from baseline, one month and six months and paired t-test was used for pairwise comparisons. The mean difference with its 95% confidence interval was calculated. The percentage loss was also calculated. The p-value < 0.05 was considered significant. All the analysis was carried out using spss 15.0 version.
Results
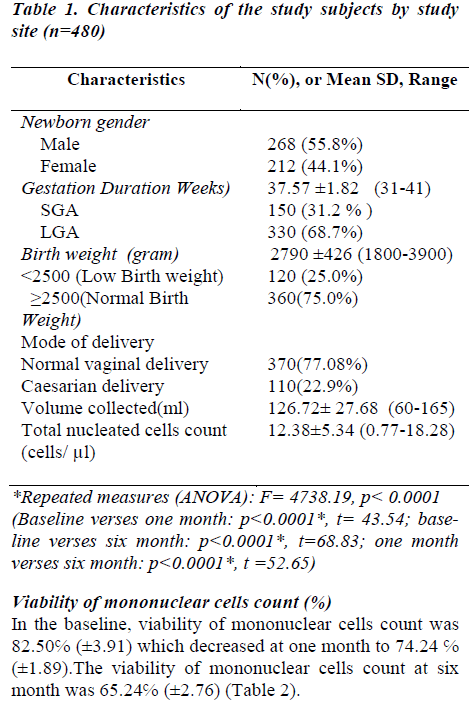
A total of 480 samples of UCB were analyzed. Considering newborn sex distribution, out of 268 infants (55.8%) were males and 212 (44.6%) were females. The median gestational age was 38 wks (mean 37.57 ±1.82 wks, range 31 – 41).Out of 480 cord blood donors 150 (31.2%) females were of small gestational age (SGA) and 330 (68.7%) were of large gestational age (LGA). The distribution of birth weight was normal (mean 2790 ± 426 gram, range 1800-3900). 120 (25.0%) baby have weight below 2500 and 360 (75.0%) baby have 2500 to 3900 gram (Table-1).
CD34+ cell concentration (%)
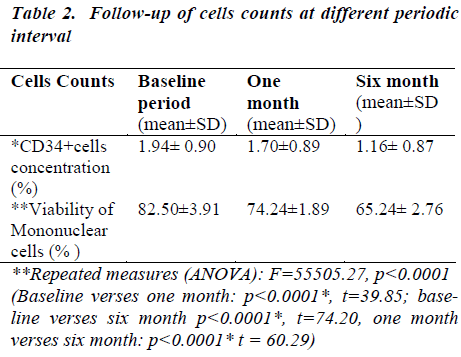
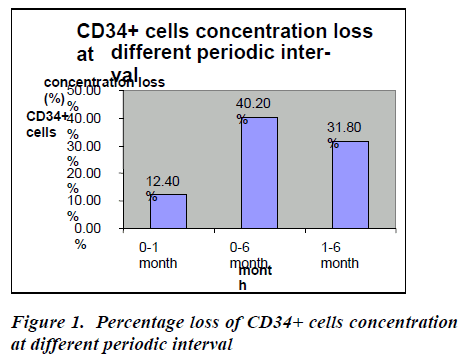
In the baseline, CD34+ cells concentration was 1.94 ℅ (±0.90) which decreased at one month to 1.70 ℅ (±0.89) which decreased at six months to 1.16℅ (±0.87) (Table- 2). After thawing and washing the mean CD34+ cell loss from base line to one month was found to be 0.24℅ (95% CI: 0.23-0.25), which was statistically significant (p<0.0001*). This loss was 12.4% which is shown figure- 1. The mean CD34+ cell loss baseline verses six months was found to be 0.78℅ (95% CI: 0.76-0.80) which was also statistically significant (p<0.0001*). This loss was 40.2% (figure-1). The mean cell loss from one month to six months was 0.54℅ (0.52-0.56%) and this loss was 31.8% (figure-1) and it was found to be statistically significant (p<0.0001*)
Viability of mononuclear cells count (%)
In the baseline, viability of mononuclear cells count was 82.50℅ (±3.91) which decreased at one month to 74.24 ℅ (±1.89).The viability of mononuclear cells count at six month was 65.24℅ (±2.76) (Table 2).
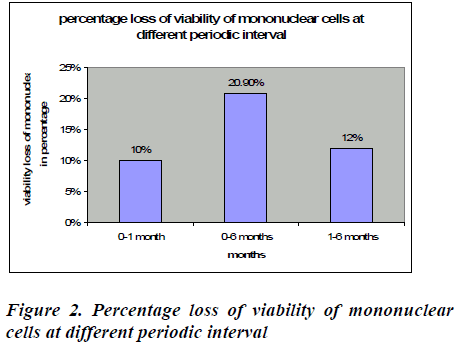
After removal of cryopreservative solution (DMSO + human albumin) the mean viability of mononuclear cell loss from base line to one month was found to be 8.26℅ (95% CI: 7.85-8.67), which was statistically significant (p<0.0001*). This loss was 10% which is shown in figure 2. The mean viability of mononuclear cell loss from baseline verses six months was found to be 74.20℅ (95% CI: 16.80-17.71) which was also statistically significant (p<0.0001*). This loss was 20.9%(figure 2).The mean cell loss from one month to six months was 8.99℅ (8.70-9.29) and this loss was 12% and it was found to be statistically significant (p<0.0001*) (figure 2). The mean of the total nucleated cells count before RBC depletion was 12.38±5.34 cells/μl (range0.77-18.28). The mean of the total volume of cord blood collection was 126.72± 27.68 (range60-165) (Table 1).
Discussion
Umbilical cord blood has been recently considered a useful alternative source of hematopoietic progenitor cells for clinical application [11]. The principal limitations of allogenic HSC transplantation are the lack of suitable HLA matched donors and complication of graft versus host disease. Although there are currently more than 1.5 million HLA - A, B and DR typed marrow donors registered in marrow donor registries worldwide, 50% of all patients requiring transplant therapy are still unable to find a suitably matched donor [12]. To alleviate a shortage of suitable donors and reduce the length of the bone marrow donor search process, Rubinstein et al (New York) initiated Placental Blood Banking Programs [13]. UCB is abundantly available and easy to collect, and frozen cord blood is immediately available for transplantation. When establishing large cord blood banks, it seems possible to balance common and uncommon HLA types, thus including minorities who are poorly represented within registries of bone marrow donors in adults. Thus, UCB is a novel and unique source of transplantable stem cells that can be used for treatment of diseases that normally require bone marrow transplantation. UCB, which is normally discarded, can be readily collected without danger to the mother or infant and the technical feasibility of using umbilical cord blood for transplantation has been established. In the unrelated cord blood transplant setting, despite the higher HLA permissiveness, a large number of stored units are required and, consequently, the development of cord blood banks is necessary. The New York Blood Center has been a pioneer in this field. In 1994 Kurtzberg et al reported the first two unrelated cord blood transplants, and to date more than 700 related and unrelated cord blood transplants have been performed [14]. Successfully hematopoietic and immunological engraftment can occur when UCB is the source of stem cells, even in cases of HLA antigen disparity between donor and recipient [15]. As a result of these transplants more cord blood banks are being set up in Europe (London, Milan, Düsseldorf etc) as part of the European Cord Blood Bank Project (Eurocord), with more than 40000 units currently stored all over the world [16]. A number of different procedures have been proposed for UCB collections, including open systems in which cord blood is collected by gravity in bottles or plastic bottles or closed systems in which modified blood collections are Iuns eodu. r study we have used a closed system that allows an average volume of cord blood collection of 126.72 ± 27.68 ml (range 60-165) The mean of the total nucleated cells count was 12.38± 5.34 cells/μl, (range 0.77-18.28). Another study shows that the closed system allows an average collection of 101.33 ml (range, 65-140ml) of cord blood [17] and they also found that average nucleated cells count/ml of cord blood was 13.97x107 with a range of 4.8 to 27.2x107. While others authors also used closed system and found that the mean of the total volume of cord blood collection was 84.6±23.6 ml and the mean of the total nucleated cells count was 0.90 ± 0.37x109 cells/ml [18].
The amount of fetal blood remaining in the placenta and the umbilical cord after clamping and dissection depends on several factors. The technique of umbilical cord blood collection varies between different cord blood banks [19]. In our study umbilical cord blood was collected before placental delivery. Reboredo et al collected cord blood before and after the placenta delivery. The collection from the placenta in utero was easy and did not disturb the natural course of birth or the postpartum period [18]. Several centers have used separation methods for umbilical cord blood prior to cryopreservation, and good recoveries have been obtained following HES sedimentation [20], 3% gelatin sedimentation [21] or Ficoll density gradients [7]. Apart from the methods described by Sousa et al (1997) the manipulations are performed in open systems, incompatible with normal blood banking procedures [22]. When the method does not involve a closed bag system of separation, the risk of microbial contamination is increased. In the present study we have used separation methods for UCB prior to cryopresevation and RBC depletion was done by using HES sedimentation. In comparison with other methods of sedimentation, HES 6% in NaCl does not require any laboratory preparation since it is commercially available and it can be used in a closed system, thus impeding possible microbial contamination during handling. It is frequently used in surgery for volume replacement [23] and is licensed in many countries for RBC removal from bone marrow to be used for transplantation [24]. Cryo preservatives are necessary additives to stem cell concentrates, since they inhibit the formation of intra and extra cellular crystals and hence cell death. The standard cryoprotectant is DMSO, which prevents freezing damage to living cells [25]. It was initially introduced into medical use as an anti-inflammatory reagent and is still occasionally used in autoimmune disorders [26]. In present study we used DMSO at a concentration of 10% as cryoprotectant with normal saline and serum albumin which is similar to other studies and this was established to be safe and non stem toxic agent [27].
Some groups elect to add a large molecular weight, non-permeable CPA as well, such as dextran or hydroxyethyl starch (HES). The permeable CPAs such as DMSO protect not by specific chemical action but by their colligative properties [28] to reduce the fraction of the cellular constituents and extracellular solution frozen at any given temperature, suppress the salt concentration in solution, and reduce harmful cell shrinkage at any given temperature. Some authors published a study in which they found that CD34+ cell concentration decreased after thawing and increased after washing, although the differences we observed were not significant. The recovery of CD34+ cells was 97 percent (95% CI, 50.1%-143.1%) post thaw and 148.9 percent (95% CI, 112.8%-185%) post wash [29]. In the present study, we found that the mean CD34+ cell loss from base line to one month was found to be 0.24℅ (95% CI: 0.23-0.25), baseline verses six month was found to be 0.78℅ (95% CI: 0.76-0.80%) and one month verses six month was 0.54℅ (0.52-0.56 %.). These losses were 12.4℅, 40.2℅, 31.8℅ from base line to one month, baseline verses six month and one month to six month respectively. All are statistically significant (p< 0.0001). Greatest cell loss occur from baseline to six months.
Some authors shows that viability, when assessed by AO/PI staining, decreased after thawing, from 97 percent (95% CI, 95%-99%) pre freeze to 62 percent (95% CI, 54%-69%) post thaw (p < 0.001). There was no significant impact of washing on viability [30].In our study viability of mononuclear cell was assessed by Trypan blue dye exclusion test and we found that the mean viability of mononuclear cell loss from base line to one month was found to be 8.26℅ (95% CI: 7.85-8.67), baseline verses six months was found to be 74.20℅ (95% CI: 16.80- 17.71) and one month verses six months was 8.99℅ (95% CI: 8.70-9.29). These losses were 10℅, 40.2℅, 31.8℅ from base line to one month, baseline verses six months and one month to six months respectively. All are statistically significant (all are p<0.0001). In here, we describe a simple and effective system for UCB processing in a triple bag, which removes 80.8 ± 5.8% of RBC and allows the storage of UCB units in small volumes, thus reducing the cost of large-scale UCB banking. In this study we have used separation methods for UCB prior to cryopreservation, and good recoveries have been obtained following HES sedimentation (6% HES in 0.9 NaCl).
To conclude the total nucleated cells count and CD34+cells count was of primary importance in graft selection so that concern for loss of cells due to wash step is recommended. We found that there was a significant effect of UCB processing on the CD34+ cells concentration and viability of mononuclear cells count. Our study also shows that after six months of cryopreservation, the amount of viable CD34+ cells were within an adequate range needed for successful engraftment.
We believe that our method revealed the processing of UCB units can be done in a closed system that also achieved a significant reduction in storage needs and related costs, while maintaining quantity and quality of the hematopoietic stem cells.
Acknowledgements
W would like to thanks all the junior resident and staff nurses of the Department of Obstetrics and Gynaecology department for collection of umbilical cord blood. This research work was supported by a Grant from the Council of Science and Technology, Uttar Pradesh, Lucknow, India.
References
- Woodsa EJ, Pollok KE, Byersa MA, Perrya BC, Purttemanc J, Heimfeldd S, Gao D et al. Cord Blood Stem Cell Cryopreservation.Transfus Med Hemother 2007; 34: 276-285.
- Wagner JE, Kernan NA, Steinbuch M, Broxmeyer HE , Gluckman E. Allogeneic sibling umbilical cord blood transplantation in children with malignant and nonmalignant disease.Lancet1995; 346: 214-219.
- Rubinstein P, Rosenfield RE, Adamson JW, Stevens CE. Stored placental blood for unrelated bone marrow reconstitution. Blood 1993; 81: 1679-1690.
- Rubinstein P. Why cord blood? Hum Immunol. 2006; 67: 398-404.
- Harris DT, Rogers I. Umbilical cord blood: a unique source of pluripotent stem cell for regenerative medicine. Curr Stem Cell Res Ther 2007; 2: 301-309.
- David Berz, Elise M. McCormack, Eric S. Winer, Gerald Colvin, and Peter J. Quesenberry, Cryopreservation of Hematopoietic Stem Cells.American Journal of Hematology 2007; 82: 463-472.
- Harris DT, Schumacher MJ, Rychlik S, Booth A, Acevedo Collection, separation and cryopreservation of umbilical cord blood for use in transplantation. Bone Marrow Transplant 1994; 13: 135-143.
- Nagler A, Peacock M, Tantoco M . Separation of hematopoietic progenitor cells from human umbilical cord blood. J Hematother 1993; 2: 243-245.
- Rubinstein P, Dobrila L, Rosenfield R, Adamson JW, Migliaccio G, Migliaccio AR, Taylor PE, Stevens CE. Processing and cryopreservation of placental/umbilical cord blood for unrelated marrow reconstitution. Proc Natl Acad Sci USA 1995; 92: 10119-10122.
- Armitage S, Fehily D, Dickinson A, Chapman C, Mavarrete C, Contreras M. Cord blood banking:volume reduction of cord blood using a semi-automated closed system. Bone Marrow Transplant 1999; 23: 505-509.
- Thompson C. umbilical cords: turning garbage into clinical gold. Science 1995; 268: 805-806.
- Confer DL. Unrelated marrow donor registries. Curr Opin Hematol 1997; 4: 408-413.
- Rubinstein P, Adamson JW, Stevens C. The placental umbilical cord blood program of the New York Blood Center. A progress report. Ann NY Acad Sci 1999; 30:328-336.
- Kurtzberg J, Graham ML, Casey J. The use of umbilical cord blood in mismatched related and unrelated hemopoietic stem cell transplantation. Blood Cells 1994; 20: 275-284.
- Rubinstein P, Carrier C, Scaradavou A et al. Initial results of the placental umbilical cord blood program for unrelated bone marrow reconstitution. New Engl J Med 1998; 339: 1565-1577.
- Rebulla P, Lecchi L, Porretti L et al. Practical placental blood banking. Transfus Med Rev 1999; 13: 205-226.
- Col PS Dhot, Maj D Sirohi, Brig GLN Swamy. Collection Separation, Enumeration and Cryopreservation of Umbilical Cord Blood Haematopoietic Stem Cells - An Experimental Study MJAFI 2003; 59:54.
- N M-Reboredo, A Diaz, A Castro and RG Villaescusa Collection, processing and cryopreservation of umbilical cord blood for unrelated transplantation. Bone Marrow Transplantation 2000; 26: 1263-1270.
- Bertolini F, Lazzari L, Lauri E, Corsini C, Castelli C, Gorini F. Comparative study of different procedures for the collection and banking of umbilical cord blood. J Hematother 1995; 4: 29-36.
- Bertolini F, Battaglia M, Zibera C, Baroni G, Soro V, Perotti C, Salvaneschi L. A new method for the placental/ cord blood processing in the collection bag. I.Analysis of factors involved in red blood cell removal.Bone Marrow Transplant 1996; 18: 783-786.
- Nagler A, Peacock M, Tantoco M, Lamons D, Okarma T.B., Lamons D. Separation of hematopoietic progenitor cells from human umbilical cord blood. J Hematother 1993; 2: 243-245.
- Sousa T, de Sousa ME, Godinho MI. Umbilical cord blood processing: volume reduction and recovery of CD34i cells. Bone Marrow Transplant 1997; 19: 311- 313.
- Stehling LC. Volume replacement in the surgical patient. In: Rossi EC, Simon TL, Moss GS (eds). Principles of Transfusion Medicine. Williams and Wilkins: Baltimore, 1991, pp 429-434.
- Walker RH. Technical Manual of the American Association of Blood Banks. AABB: Arlington, VA, 1990; pp 646-647.
- Lovelock JE, Bishop MW. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature 1959; 183: 1394-1395.
- Albanell J, Baselga J. Systemic therapy emergencies. Semin Oncol 2000; 27: 347-361.
- Branch DR, Calderwood S, Cecutti MA, Herst R, Solh H. Hematopoietic progenitor cells are resistant to dimethyl sulfoxide toxicity. Transfusion 1994; 34: 887-
- 8S9k0o.r ic D, Balint B, Petakov M, Sindjic M, Rodic P: Collection strategies and cryopreservation of umbilical cord blood. Transfus Med 2007; 17: 107-113.
- Laroche V, McKenna DH, Moroff G, Schierman T, Kadidlo D, McCullough J. Cell loss and recovery in umbilical cord blood processing: a comparison of postthaw and postwash samples. Transfusion 2005; 45: 1909.
- Canabarro R, Sporleder H, Gomes T, Zanatta G, Scribe L, Freitas F, Neumann J, Pranke P. Immunophe notypic evaluation, and physiological and laboratory correlations of hematopoietic stem cells from umbilical cord blood. BIOCELL 2007; 31(3):397-303.