ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2010) Volume 21, Issue 4
Evaluation of random donor platelets at different temperatures for an ex-tended shelf life
1Department of Transfusion Medicine and Blood Bank
2Department of Pathology, Chhatrapati Shahuji Maharaj Medical University, Lucknow, Uttar Pradesh, India.
- *Corresponding Author:
- Tulika Chandra
Department of Pathology and Blood Bank
Chhatrapati Shahuji Maharaj Medical University
Lucknow 226003, Uttar Pradesh,India
Phone: +91-9415755536
E-mail: tulikachandra@rediffmail.com
Accepted May 03 2010
Platelet concentrates can be stored for five days at 22 0C. Our objective was to increase the storage of platelets to 7 days by decreasing the storage temperature. Lower tempera-ture may minimize chances of bacterial proliferation and also maintain platelet func-tions to optimum level. The study sample iincluded 25 blood donors of both sex in State Blood Bank, Chhatrapati Shahuji Maharaj Medical University, Lucknow. Complete his-tory of donors was taken to exclude any infection and disease. The platelet concentrates were prepared by platelet rich plasma (PRP) method. The whole blood (350 ml.) was col-lected in anticoagulant Citrate Phosphate Dextrose Adenine (CPDA) triple blood bags. Random donor platelet concentrates were evaluated on day 0, day 5 and day 7 at differ-ent temperatures of storage period. Platelet swirling was present in all the units at all the temperatures on day 7 with no evidence of bacterial contamination. Comparison of the mean values of platelet count, PF 3, lactate dehydrogenase, pH, glucose and platelet ag-gregation, showed no significant difference at 22 0C and 18 0C while PF 3, pH and glu-cose level showed a significant difference on day 7 at a temperature of 16 0C.
Keywords
Random donor platelet, Shelf life, Day, Temperatures
Introduction
Random donor platelets with prolonged shelf life will have logistics of increased platelet availability for pa-tients. Platelets are small, anuclear cytoplasmic fragments that play an essential role in blood clotting and wound healing. Megakaryocytes shed platelets into the blood stream where they circulate for around 10 days before being destroyed by the reticuloendothelial system, primar-ily in the liver and spleen [1]. Platelets are routinely iso-lated from whole blood, concentrated and stored in plasma for use in transfusion therapy [2]. Such platelet concentrates can be stored for five or even seven days and still be therapeutically effective in thrombocytopenic pa-tients. Platelet transfusions are effective for prevention and treatment of bleeding in patients who have quantita-tive or functional platelet disorders [3]. Transfusion effi-cacy in clinical practice for clinically stable thrombocyto-penic patients is mainly based on the quantitative increase of platelets, the functional aspect of transfused platelets not being considered. Studies conducted with platelet concentrates showed these cells lose their viability very quickly during the storage period, implying the need for a constant renewal of stock [4,5]. The present study was done to assess the in vitro functional viability of platelet concentrates stored for seven days at temperature of 22 °C, 18 °C and 16 °Cto improve the logistics of platelet availability. Lower temperature may minimize chances of bacterial proliferation and also maintain the efficacy of platelet functions at optimum level.
Material and Methods
The present study was conducted on the samples of 25 blood donors of both sex in State Blood Bank, Chhatra-pati Shahuji Maharaj Medical University, Lucknow. Complete medical history of donors was taken to exclude any infection and disease in the collected samples.
Subjects Studied
The blood donors were selected after a complete medical history and examination. Only those donors who were absolutely healthy and free from any disease were in-cluded in the study. Written consent of the donors was taken regarding the acceptability for the tests to be carried out for the transfusion transmitted diseases as well for the platelet function studies.
A) Platelet Isolation
The platelet concentrates were prepared by platelet rich plasma (PRP) method [6]. The whole blood (350 ml.) was collected in anticoagulant Citrate Phosphate Dextrose Adenine (CPDA) triple blood bags (HL Hemopack, Hindustan Latex Ltd. Kerala, India). After a resting time of 30 minutes, the whole blood was centrifuged in a Cryo-fuge 6000i (Heraeus, Germany) at 1750 x g for 8 minutes at 22 °Cto obtain platelet rich plasma (PRP). The ob-tained PRP was again centrifuged at 3850 x g for 8 min-utes under same experimental conditions. After the final centrifugation the supernatant platelet poor plasma (PPP) was separated, and the residual pellet with the platelets was resuspended in a mean volume of 50 ± 0.9 ml of re-spective plasma. The platelet concentrate was divided into three parts by a sterile tubing welder (Terumo TSCD, SC-201 AH, Leuven, Belgium). The bags were placed in a platelet incubator with agitator (Remi Instruments Ltd., Mumbai, India). The platelet concentrates were evaluated on day 0, day 5 and day 7 at different temperatures of storage.
B) Screening of blood and Storage of platelet units
All the blood units were screened for Hepatitis B Virus, Hepatitis C Virus, Human Immunodeficiency Virus 1 and 2. Method used was Elisa (Elisa plate washer version 3 and Elisa plate reader version no. 1.300, Robonik Pvt. Ltd., Navi Mumbai, India). Syphilis was tested by Rapid Plasma Reagin (RPR) method (Span Diagnostic Ltd., Su-rat, India). Platelet concentrates were stored at 22 °C, 18 °C and 16 °C in different platelet incubators and agitators. 25 units each of platelet concentrates were stored at 22 °C, 18 °C and 16 °C.
C) Assessment of Platelet Count and Functions
Standard protocols were followed to perform a quantita-tive and qualitative analysis of platelets on day 0, day 5 and day 7 of storage at all the temperatures. Samples were withdrawn under sterile conditions in biosafety cabinet grade 2. Platelet count was done by automated cell counter (MS4, Blood cell counter, Anand Group, HD Consortium, India). Platelet functions were assessed by Platelet Factor 3 (PF-3) with Kaolin and CaCl2. This was done as described by Hardisty et al [7]. Lactate Dehydro-genase (LDH) estimation was done on these samples. Platelet concentrate (1 ml) was centrifuged at 3000 x g for 5 minutes. The supernatant was used to quantify the LDH by Semi auto analyzer, Mumbai, India. Glucose determi-nation was done by centrifuging 2 ml of platelet concen-trate in fluoride oxalate vial at 3000 x g for 5 minutes. The supernatant was used to quantify the glucose by Er-bachem 5 Plus analyzer (Erba diagnostic Mannhein Gmbh, Mannhein, Germany). pH of all samples was as-sessed immediately after sampling at a temperature of 24 °Cby Compla pH meter (Composite Lab Line Pvt. Ltd, Lucknow, India). Aerobic culture was performed on all the samples on day 0, day 5 and day 7 using manual method of culture [8]. The readings at day 5 and day 7 were analyzed taking day 0 as control.
d) Statistic Analysis
Data was reported as mean ± standard deviation (SD). The data was compared using paired “t”-test. The confi-dence limit was kept at 95%, hence a “p” value <0.05 was considered to be statistically significant. difference
Result
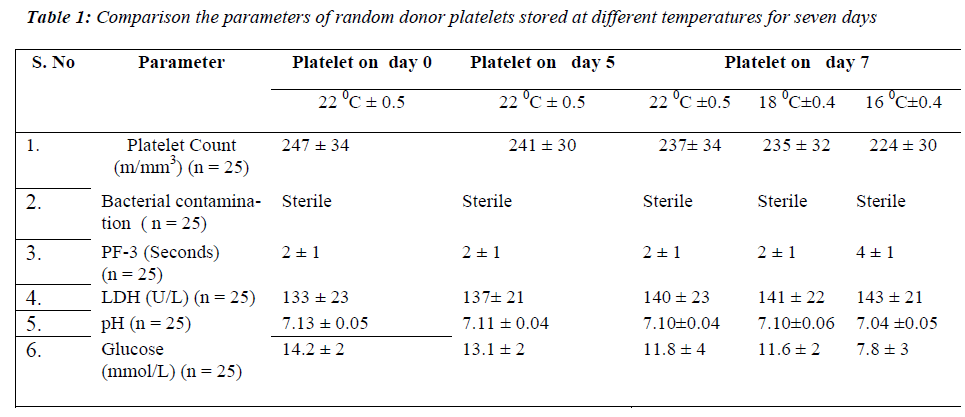
None of the samples showed bacterial contamination on day 7 at 22 °C± 0.5, 18 °C± 0.4 and 16 °C± 0.4. On comparing the mean values of platelet count at all the temperatures, no significant difference was found on day 7 of storage period. PF 3 there was no significant on day 7 at 22 °Cand 18 °C. In contrast a significant dif-ference in PF 3 values was observed on day 7 (p < 0.001) at 16 °C. The mean values of LDH level showed no sig-nificant difference on day 7 at all the temperatures of storage period. The pH showed no significant difference on day 7 at 22 °C and 18 °C but a significant decrease was observed on day 7 (p < 0.001) at 16 °C. For glucose lev-els, there was a significant decrease only on day 7 (p < 0.001) at 16 °C (Table 1).
Discussion
The three major points in the production and storage of platelet concentrates are essential to maintain good plate-let quality. First, the activation of platelets during collec-tion, preparation and storage of platelet concentrates should be prevented or at least reduced to a very low level. Secondly, the level of glycolytic activity, the an-aerobic consumption of glucose and production of lactate, should be kept to a minimum level. Thirdly, at least some glucose should be present in the platelet concentrates throughout the whole storage period [9]. Platelet concen-trates can be prepared by random donor platelets, aphere-sis and by pooling of platelet units [10]. Random donor platelets were used in the present study. According to guidelines of blood bank, random donor platelets are stored for five days at 22 °C [11]. Due to these storage conditions, platelets have limited availability. We there-fore carried out a study investigating random donor plate-lets stored at lower temperature to evaluate the influence of prolonged storage on platelet function and metabolism and minimizing the chances of bacterial proliferation at lower temperature. In the present study, platelet swirling was present in all the units at 22 °C, 18 °C and 16 °C on day 7. Temperature below 15 °C causes resting platelets to rapidly change from disc to spidery forms [12]. The reduction in viability after storage at lower temperature correlates with reduction in number of discoid platelets. Hence the temperature of 16 °C was chosen in order to eliminate the above factors. No evidence of bacterial con-tamination was found on day 7 at all the temperatures. In the present study, platelet count was maintained on day 7 at all the temperatures.
Platelets are an important source of phospholipids for the intrinsic process of blood coagulation, and these phos-pholipids become available during the release reaction as ‘Platelet Factor 3’. Further information can be obtained by examining the release of PF 3 into supernatant plasma during the clumping of the platelets in an aggregometer [13]. PF 3 may also be released by antiplatelet antibodies. However, the kaolin test has the important advantage of extreme simplicity both in apparatus and in performance. The two mixtures (Platelet rich test plasma and Platelet poor normal plasma) differ only in the platelet they con-tain and clotting time should not differ by more than 2 or 3 seconds. A prolongation of clotting time of the mixture containing the test platelet compared to that containing the normal platelets is an evidence of reduced PF 3 avail-ability. It is desirable to measure the clotting time of mix-ture of platelet rich and platelet poor sample of the test plasma and normal plasma respectively. The present study showed PF 3 variation within 3 seconds at all tem-peratures even on day 7. This test has been previously described by Requejo PJL [14]. The fact that platelet fragment can retain their procoagulant activity lends cre-dence to the commonly held theory that PF 3 generation is dependent on configurational changes of the platelet membrane [15].
In the present study LDH level increased and was main-tained on day 7 at 22 °C, 18 °C and 16 °C. It is essential that during storage the pH level of platelet concentrates remains within acceptable range of 6.4 -7.4 in order to retain the platelet functions. The present study showed that pH value decreased but was maintained within ac-ceptable range on day 7 at all the temperatures of the stor-age period.
In the present study it was observed that the glucose level was decreased but maintained on day 7 at 22 °C and 18 °C. In contrast it changed significantly at 16 °C on day 7 of storage period. There is ample evidence that platelets can oxidize fatty acids [16]. Furthermore, plasma free fatty acids actually increase during storage [17] so ade-quate levels are present for metabolism.
The percent change of platelet functions at 16 °C is higher as compared to the temperature at 18 °C and 22 °C. In glucose decrease in value is 45% at 16 °C which is in marked contrast to the temperature at 18 °C (18.3%). It is probably due to the increased catabolism of the cells at 16 °C [18]. pH value was 1.2% at 16 °C due to increased ca-tabolism of cells leading to cell acidity. PF 3 value in-creased at 16 °C signifying that the release activity detori-ates with time due to change in the morphology of plate-lets.
The overall result showed that random donor platelet con-centrates stored for days 7 at 22 °C, 18 °C and 16 °C var-ied in platelet functions but they were maintained best at 22 °C and 18 °C on day 7. Schlenke et al 2006 [19] have already observed the storage of 12 unit of SDP and 12 unit of buffy coat platelets till day 8 at 22 °C. The plate-lets were stored for up to day 8 and evaluated using a panel of in vitro parameters including volume, platelet count, LDH, glucose, pO2 and pCO2. Platelet swirling was present with no bacterial contamination. They found that the LDH, pO2, significantly increased while glucose and pCO2 decreased significantly in both groups on the day 8 of storage at 22 °C. Similarly, Gottschall et al 2003 [20] reported that all the platelet concentrates from normal donors were stored for 3 days under identical conditions except for the temperatures of storage, which were main-tained at 21°C ± 0.5, 19.5 °C ± 0.5, 18 °C ± 0.5 respec-tively. Immediate posttransfusion recovery of the stored platelets determined by 51Cr labeling averaged 47, 47 and 48 percent after storage at 21°C, 19.5 °C and 18 °C re-spectively (differences not significant). Mean life span of the transfused platelets, however, was 8.12, 5.12, and 1.85 days at 21°C, 19.5 °C and 18 °C respectively. The differ-ence between mean life span following storage at 21°C was significantly difference after storage at 18°C (p less than 0.03). They found that the platelet viability is com-promised after storage for 3 days at 18°C and, possibly 19.5 °C. The parameters analyzed showed that reduction in viability after storage at the lower temperature corre-lated with the reduction in the number of discoid platelets.
Our study infers that platelet functions are maintained within normal levels at day 7 storage at 22 °C and 18 °C but not at 16 °C. No bacterial contamination was reported in any of the cases. Thus we may conclude that platelet concentrates stored at 22 °C and 18 °C may be used with an extended shelf life of 7 days. This will lead to a tre-mendous increase in the stock position of platelets thus making them easily available to the patients. Further in vivo studies are needed to change the protocols for plate-lets shelf life.
Conclusion
Our study infers that platelet functions are maintained within normal levels on day 7 of storage at 22 °C and 18 °C but not at 16 °C. Thus we may conclude that platelet concentrates storage can be extended to 7 days at a tem-perature of 18 °C.
References
- Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA. Programmed anuclear cell death delimits platelet life span. Cell 2007; 128: 1173-1186.
- Akay OM, Gunduz E, Basyigit H, Gulbas Z. Platelet function testing during five day storage of single and random donor platelet pheresis. Transfus Apher Sci 2007; 36: 285-289.
- Fabris F, Soini B, Sartori R, Randi ML, Luzzatto G, Girolami A. Clinical and laboratory factors that affect the post-transfusion platelet increment. Transfus Sci 2000; 23: 63-68.
- Wildt-Eggen J, Schrijiver JG, Bins M, Gulliksson H. Storage of platelets in additive solution: effect of mag-nesium and/or potassium. Transfusion 2002; 42: 76-80.
- Gulliksson H. Platelet storage. Transfus Apher Sci 2001; 24: 241-244.
- Tyler V, Brecher M, Pisciotto P, Pierce S, editors. Technical American Association of Blood Banks Man-ual, 13th ed. Bethesda: Maryland, USA 1999; 1-798.
- Hardisty RM, Hutton RA. The Kaolin Clotting time of PRP: A test of PF-3 aviability. Br J Hematol 1965; 11: 258-260.
- Collee JG, Fraser AG, Marmion BP, Simmons A. Prac-tical medical microbiology; (14th ed): Churchill, Liv-ingstone, 1996.
- Gulliksson H. Defining the optimal storage conditions for the long term storage of platelets. Transfus Med Rev 2003; 17: 209-215.
- Saran RK. Transfusion medicine technical manual; (2nd ed): WHO; New Delhi, India, 2003.
- American Association of Blood Bank. Standards for blood banks and transfusion services; (23rd ed): Be-thesda, Maryland, 2004.
- Winokaur R, Hartwig JH. Mechanism of shape change in chilled human platelets. Blood 1995; 85: 1796-1804.
- Hardisty RM, Hutton RA. Platelets aggregation and the availability of platelet factor 3. Br J Hematol 1966; 12: 764-768.
- Requejo PJL. A Standardized bioassay for platelet fac-tor 3 released by kaolin. Br J Hematol 1976; 33: 39-51.
- Filip DJ, Eckstein JD, Sibley CA. The effect of platelet concentrates storage temperature on adenine nucleotide metabolism. Blood 1975; 45: 749-56.
- Cohen P, Wittels B. Energy substrate metabolism in fresh and stored human platelets. J Clin Invest 1970; 49: 119-122.
- Hamid MA, Kunicki TJ, Aster RH. Lipid composition of freshly prepared and stored platelet concentrates. Blood 1980; 55: 124-126.
- Amorini AM, Tuttobene M, Lazzarino G. Evaluation of biochemical parameter in platelet concentrates stored in glucose solution. Blood Trasnsfus 2007; 5: 24-32
- Schlenke P, Franck V, Lin L, Kirchner H. Storage of apheresis and pooled buffy coat platelet concentrates treated with pathogen inactivation using the intercept blood system. I S Blood Trans 2006.
- Gottschall JL, Rzad L. Studies of the minimum tem-perature at which human platelets can be stored with full maintenance of viability. Transfusion 2003; 26: 460-462.