ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2022) Volume 33, Issue 3
Evaluation the effect of metformin on proliferation, neurotrophic expression and antioxidant activity of mesenchymal stem cells
Samyra Sistani, Abootaleb Kosha, Mohammad Taghi Ghorbanian*
Department of Biology, Damghan University, Damghan, Iran
- Corresponding Author:
- Mohammad Taghi Ghorbanian
Department of Biology
Damghan University
Damghan
Iran
Accepted date: March 3, 2022
Metformin is considered cheap, safe and efficient medication worldwide, which is used as a first-line treatment for type 2 diabetes, has exhibited great interest for its potential antiaging properties. There is increasing evidence that metformin can be used in a variety of therapeutic conditions due to its biological effect. Much attention has been paid to improving culture conditions, increasing the capacity and capability of Mesenchymal Stem Cells (MSCs). In this study, the viability and cell proliferation rate, antioxidant enzyme activity‚ and expression of neurotrophic genes at passage 3 of metformin-treated rat Bone marrow Mesenchymal Stem Cells (BMSCs) were evaluated.
BMSCs were isolated and treated with 1, 5, 10, 15, and 50 μM of metformin for 24 h. Then the survival rate of cells was measured by MTT assay. The growth rate and proliferation of cells at 24 hours after culture were assessed by Bromodeoxyuridine (BrdU) markers. Superoxide Dismutase (SOD) enzyme activity, Glutathione Peroxidase (GPX), and Malondialdehyde (MDA) levels were measured. Expression of neurotrophic factors Brain-Derived Neurotrophic Factor (BDNF), Glial cell-line Derived Neurotrophic Factor (GDNF) and Neurotrophin-3 (NT3) were also determined by Reverse Transcription Polymerase Chain Reaction (RTPCR).
The results of this study indicate that the proliferation rate by BrdU markers at 5 and 15 μM metformin showed a significant increase compared to control groups (P<0.5). Gene expression density of neurotrophic factors (NFs) showed that, there were significant differences between MSCs treatment groups and control group (P<0.5). Also, metformin-treated cell groups showed a higher antioxidant capacity than the control group. Metformin may be suggested as a pre-therapist to strengthen mesenchymal stem cells before transplantation for the treatment of neurodegenerative diseases.
Keywords
Metformin, Mesenchymal stem cells, Cell proliferation, Antioxidant.
Introduction
Metformin is a considered cheap, safe and efficient medication worldwide, which is used as a first-line treatment for type 2 diabetes, has exhibited great interest for its potential antiaging properties [1]. There is increasing evidence that metformin can be used in a variety of therapeutic conditions due to its biological effect [2]. While metformin is mainly used for the treatment of diabetes, numerous studies have shown that it may have further application in anticancer and antiaging therapies [3]. Several studies have shown that metformin affects the proliferation of stem cells and neurogenesis that could be associated with increasing neurotrophic factors; therefore it can be effective in neurodegenerative diseases such as Alzheimer’s and Parkinson’s [4-6] .In the field of stem cell medicine, major scientific interest is focused on the cytophysiological characteristics and clinical use of Mesenchymal Stem Cells(MSCs). MSCs have several properties to use in tissue engineering, including: proliferation ability and differentiation capacity and neuroprotective properties mediated by secretion of various neurotrophic factors, and immunomodulatory, anti-apoptotic, and anti-inflammatory effects. MSCs possess the abilities of cell engraftment, and in addition, their easy accessibility and quick in vitro expansion make MSC an ideal resource for regenerative transplantation therapy [7,8].
Reactive oxygen species are the products of aerobic metabolism that regulate different cellular stages including apoptosis‚ differentiation and, aging [9]. Oxidative stress means an imbalance between the production of oxidant and their elimination system‚ i.e., antioxidant enzymes [10]. The body’s biological system has an advanced mechanism to control Reactive Oxygen Species (ROS) levels‚ that neutralizes the excessive ROS and prevents cell damage. In common, increased reactive oxygen species inhibit stem cell proliferation, increase cell senescence, and inhibit immunomodulation. Moreover, cell senescence, and oxidative stress reduce their in vitro and in vivo expansion, which is critical for their stem cell transplantation [11].
Neurotrophic Factors (NFs) are large polypeptides that contribute to development and survival of the central and peripheral nervous systems [12]. At first‚ GDNF was used to increase the survival of dopaminergic neurons in the midbrain also, it can use to treat Parkinson’s patients whose dopamine neurons are damaged [12]. In Alzheimer’s disease‚ the BDNF mRNA reduces in the hippocampus and parietal cortex [13]. It has been shown that the drug induced expression of Brain-Derived Neurotrophic Factor (BDNF) and Glial cell-line Derived Neurotrophic Factor (GDNF) in mouse substantia nigra [14]. Metformin is able to increase proliferation in neural stem cells and periodontal ligament stem cells. However, the antioxidant effects of metformin against oxidative stress and its underlying mechanisms in stem cells have not been studied well. The aim of this work was to evaluate the effect of metformin on cytophysiology properties of Bone marrow Mesenchymal Stem Cells (BMSCs). Our research included cell proliferation activity, amount of oxidative stress and, neurotrophic genes expression.
Materials and Methods
Ethical approval
The study was conducted with the approval of the Bioethics Committee, as stated by the Local Bioethics Committee at the molecular and cellular department, School of Biology, Damghan University, Iran (1397/4558). Adult male Wistar rats weighing between 200 and 250 g were purchased from Razi Institute, Karaj, Iran. They were kept under standard laboratory conditions with a 12 h light/12 h dark cycle and ad libitum food and water throughout the experiments.
Preparation and culture of MSCs
Rat MSCs were obtained from the bone marrow of femurs and tibias using the method of Azizi et al. [15]. The cells were cultured in Alfa dulbecco’s Minimal Essential Medium (α-MEM), (Gibco) supplemented with 10% Fetal Bovine Serum (FBS) (Gibco), 1% penicillin and streptomycin (Gibco). The cells were seeded in 25 cm2 tissue culture flasks (Falcon) and incubated at 37ºC and 5% CO2. After 48 hr non-adherent cells were removed by replacing the medium. After the cultures reached the optimum confluence, cells were lifted by incubation with 0.25% trypsin and 0.02% EDTA (Merck) at 37ºC for 3-4 min and the subculture of the cells were re-plated at a density of 4 × 105 cells/cm2. Cell density (cell count) and cell viability were determined using a neubauer hemocytometer as explained by Davis JM and MTT assay [16-18].
Cell viability assay (MTT) [3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyl-2H-tetrazolium bromide]
Briefly 2 × 104 cells at passage 2 per well were seeded in 96-well plates, and incubated in 200 ml a-MEM supplemented with 10% FBS. After 48 hours of incubation‚ the cells were washed with PBS and then 200 μl α-MEM and different concentration of metformin (Shahrdaru, Iran) including 1‚ 5‚ 10‚ 15 and 50 μM were added to induce BMSCs. After 24 hours of treatment‚ the medium was removed and replaced with 10 μl MTT (5 mg/ml‚ Sigma) and kept at 37ºC for 4 hours. In order to form the formazan crystals‚ absorbance was read at 540 nm by an Eliza reader (Bio Tek‚ USA). The test was repeated five times for each group [19].
To identify and Cell proliferation rate BMSCs, they were placed on gelatinized slides at 5 × 104 cells/ cm2. For immunostaining, they were fixed in 4% PFA (paraformaldehyde) for 20 min at 4ºC before being permeabilized in 0.3% Triton X-100 for 15 min, and blocked in 10% normal goat serum for 15 min. Subsequently, the samples were incubated with primary antibodies overnight at 4ºC. Sources and dilution of primary antibodies used were as follows: mouse anti-BrdU primary antibody (Sigma-B 2531, 1:500). Afterwards, the cells were incubated with secondary antibody at 37ºC for 30 min. Secondary antibodies were Rhodamine-conjugated antimouse (1:100; Millipore, AP124R) for BrdU marker. Cells were examined using a fluorescent microscope (E600- Eclipse Nikon) equipped with a digital camera (DXM 1200 Camera Nikon Digital). The number of nuclei in positive cells (BrdU) was counted in 20 fields. A negative control was investigated in each experiment by replacing the primary. All immunocytochemical experiments were repeated over 3 times for each group.
Table 1 presents the primer sequences and product sizes. GAPDH was used as the housekeeping gene (internal control). The PCR products were dissolved in 1.5% agarose gel and visualized and photographed on a UV transilluminator (UVIdoc, EU). We used the UVIdoc software (version 12.6) to perform the quantitative evaluation of the band volumes, which were expressed as gene expressions. Each experiment was performed in triplicate. All the stages were controlled in the cDNA preparation stage by deleting the RNA sample and reverse transcriptase and in the RT-PCR stage by deleting Taq polymerase and the cDNA products.
| Gene | Primer Sequence (5'→3') | Amplicon Size (bp) | Accession number |
|---|---|---|---|
| BDNF-F | GACTCTGGAGAGCGTGAAT | 405 | NM_001270630.1 |
| BDNF-R | CCACTCGCTAATACTGTCAC | ||
| NT-3-F | AGGTCAGAATTCCAGCCGAT | 181 | NM_001270868.1 |
| NT-3-R | GTTTCCTCCGTGATGTT | ||
| GDNF-F | GACTCCAATATGCCCGAAGA | 242 | NM_019139.1 |
| GDNF-R | TAGCCCAAACCCAAGTCAGT | ||
| GAPDH-F | TGACATCAAGAAGGTGGTGAAGC | 203 | NM_017008.4 |
| GAPDH-R | CCCTGTTGCTGTAGCCGTATTC |
Table 1. The primers used for RT-PCR analysis.
Statistical analysis
All data are shown as mean ± standard error. Data were analyzed using SPSS software version 16. Analysis of the differences between data was done using one-way ANOVA test followed by Tukey test and P<0.05 was considered as a significant level.
Results
The effect of metformin on MSCs proliferation and cell viability
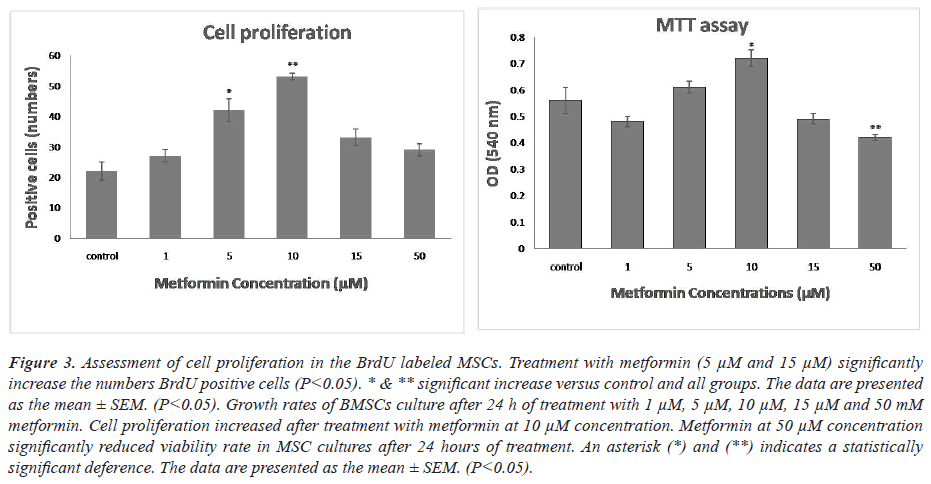
To test the antioxidant effects of metformin, BMSCs were treated with metformin for 24 hr. One-way ANOVA showed that there was a significant difference between groups in the rate of MSCs cell viability. As shown in Figures 1-3, treatment MSCs with 10 μM showed a significant increase compared to the control. Treatment with 50 μM of metformin caused a significant decrease in the proliferation rate in comparison with the control group (P<0.05).
Figure 3: Assessment of cell proliferation in the BrdU labeled MSCs. Treatment with metformin (5 μM and 15 μM) significantly increase the numbers BrdU positive cells (P<0.05). * & ** significant increase versus control and all groups. The data are presented as the mean ± SEM. (P<0.05). Growth rates of BMSCs culture after 24 h of treatment with 1 μM, 5 μM, 10 μM, 15 μM and 50 mM metformin. Cell proliferation increased after treatment with metformin at 10 μM concentration. Metformin at 50 μM concentration significantly reduced viability rate in MSC cultures after 24 hours of treatment. An asterisk (*) and (**) indicates a statistically significant deference. The data are presented as the mean ± SEM. (P<0.05).
Cells were stained for BrdU marker at 24 hours after culture. One-way ANOVA showed that there was a significant difference between groups in the rate of MSCs proliferation. Comparison of different groups of cell culture showed that BrdU positive cells were increased at induced cells with metformin in comparison with control group. MSCs at 5 and 10 μM metformin showed a significant increase of proliferation rate compared with other groups (P<0.05).
Analysis of gene expression
Our results revealed that metformin caused a significant increase in the NFs (BDNF, GDNF and NT3) expression in the treated groups compared to the control group.
The expression of the BDNF gene showed a significant increase in 5, 10 and 50 μM of metformin compared to control and various metformin concentrations‚ but no significant difference was seen between other doses of metformin (P<0.05). The expression of the GDNF gene was significantly increased in 5, 10 and 15 μM in comparison with control and other groups (P<0.05).
The comparison of semi-quantitative expression of the genes in different cell culture conditions. The comparison of gene expression in different groups showed significant difference in terms of NT3, GDNF and BDNF (P<0.05). There were significant increase of gene expression of BDNF in treatment groups (5, 10 and 50 μM) compared with others groups. There were significant increase of gene expression of BDNF in treatment groups (5, 10, 15 and 50 μM compared with others groups. There were significant increase of gene expression of NT3 in treatment groups (5 and 10 μM) compared with others groups. The level of expression of all genes was calculated in relation to the housekeeping gene, GAPDH. The data are presented as the mean ± SEM. (P<0.05).
Discussion
In fact, numerous in vivo and in vitro studies showed that metformin has multidirectional action. While metformin, as the most commonly used antidiabetic drug, it’s recognized not only as a hypoglycemic agent, but also as a drug that protects against the aging process and oxidative stress. Much attention has been paid to improving culture conditions, increasing the capacity and capability of MSCs. Possible clinical application must be based on the abundance, expansion potential and other characters of the stem cells. MSCs in vitro usually have a limited life-span and undergo senescence, indicated by the loss of proliferation ratio and altered morphology. In our previous studies the immunocytochemical testing were performed using specific markers CD71 and CD90, to identify mesenchymal cells. Also adipogenic and Osteogenic differentiation were confirmed by the formation of small lipid droplets and the production of calcium phosphate and mineralized extracellular matrix after 3 weeks. Since self- renewal is one of the common properties of MSCs, in this study BMSCs were evaluated and compared by MTT assay and BrdU marker. The BrdU marker is widely used for cell division in vitro and cell transplantation. MTT assays indicated that metformin viabilities of BMSCs in dose- dependent manners. The number of positive BrdU cells increased in 24 hours after culture with metformin. The use of BrdU marker to investigate the effect of Metformin on MSCs has been reported in a previous study. The effect of metformin concentration on stem cell proliferation has been reported differently. For example, metformin has an inhibitory effect on dental pulp cells and human chorionic villi stem cells‚ but it causes proliferation in neural and mesenchymal stem cells. Our results indicated that the rate of cell proliferation increased in a dose-dependent manner. The results of our study are in line with another study by Gao et al. showed that cells under osteogenic conditions and treated with 100 μM metformin increased proliferative and osteogenesis activity. Chang et al. results were almost in agreement with ours. A research confirmed that in 0.05 mM of metformin, the proliferation rate of Chorionic Villous Mesenchymal Stem Cells (CV-MSCs) remained unchanged, but in higher concentrations (0.1- 1 mM), cell proliferation was inhibited. Based on the discrepancy of results among studies, and according to molecular mechanisms of metformin on inhibition proliferation‚ it can speculate that the discrepancies might come from cell type and dose differences. Metformin causes cell proliferation in low concentrations‚ but it has anti-tumor effects at higher concentrations and can inhibit the proliferation of cancer cells. As shown in Figure 3, the rate of cell proliferation was significantly increased in a dose-dependent manner in the experimental groups (5 and 10 μM) at 24 h compared with the control in MSCs.
Metformin is presumed to exert their neuroprotective effects by inducing neurotrophic factor gene expression. We have found that metformin causes an increase in neurotrophic genes expression. Treated BMSCs with metformin showed about four folds higher expression of BDNF ‚GDNF and in NT3 almost two folds higher expression was seen compared to the control group. Metformin can cross the blood-brain barrier and therefore has the potential to have a direct effect on the central nervous system. Patil et al. showed that BDNF expression in rat substantia nigra increased after receiving metformin (500 mg/day) for 21 days. Moreover, it has been reported that neuroprotective effect of metformin through enhancing of expression of BDNF in Parkinsonism mice. Allard et al. reported that metformin mixed with food decreased the BDNF mRNA for 6 months. In a study by Katila et al. the neuroprotective effect of metformin on Parkinson's model induced by MPTP was investigated.The results of their study showed that metformin increased BDNF and GDNF genes expression in rat substantia nigra. As metformin activates AMPK‚ they suggested that its neuroprotective effect is associated with AMPK activation. Neurotrophic factors such as BDNF, GDNF and NT3.
Conclusion
The results of this study indicate that the self-renewal and proliferation rate by metformin showed a significant increase compared to control groups. Also, metformin- treated cell groups showed a higher antioxidant capacity than the control group. Metformin seems an appropriate inducer factor to induce neurotrophic genes such as BDNF, GDNF, and NT3 genes and therefore can be used to treat neurodegenerative diseases like Alzheimer’s. Additionally, we observed that metformin supplementation signifcantly increased neurotrophic factors in MSCs and simultaneously increased SOD and GPX activity. Another potentially important finding of this study was that metformin can ameliorate culture condition in MSCs.
Conflict of Interest
We declare there is no conflict of interest between authors.
References
- Saini N, Yang X. Metformin as an anti-cancer agent: Actions and mechanisms targeting cancer stem cells. Acta Biochim Biophys Sin (Shanghai) 2018; 50: 133-143.
[Crossref] [Google Scholar] [PubMed]
- Śmieszek A, Czyrek A, Basinska K, Trynda J, Skaradzińska A, Siudzińska A, Marędziak M, Marycz K. Effect of metformin on viability, morphology, and ultrastructure of mouse bone marrow-derived multipotent mesenchymal stromal cells and Balb/3T3 embryonic fibroblast cell line. Biomed Res Int 2015; 2015: 769402.
[Crossref] [Google Scholar] [PubMed]
- Alexander A Soukas, Haibin Hao, Lianfeng Wu. Metformin as anti-aging therapy: Is it for everyone? Trends Endocrinol Metab 2019; 30: 745–755.
[Crossref] [Google Scholar] [PubMed]
- Houshmand F, Barati M, Golab F, Ramezani-Sefidar S, Tanbakooie S, Tabatabaei M. Metformin-induced AMPK activation stimulates remyelination through induction of neurotrophic factors, downregulation of NogoA and recruitment of Olig2+ precursor cells in the cuprizone murine model of multiple sclerosis. Daru 2019; 27: 583-592.
[Crossref] [Google Scholar] [PubMed]
- Jiang LL, Liu L. Effect of metformin on stem cells: Molecular mechanism and clinical prospect. World J Stem Cells 2020; 12: 1455-1473.
[Crossref] [Google Scholar] [PubMed]
- Markowicz-Piasecka M, Sikora J, Szydłowska A, Skupień A, Mikiciuk-Olasik E, Huttunen K M. Metformin: A future therapy for neurodegenerative diseases. Pharm Res 2017; 34: 2614-2627.
[Crossref] [Google Scholar] [PubMed]
- Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: A detailed progress report of the last 6 years (2010-2015). Stem Cell Res Ther 2016; 7: 82.
[Crossref] [Google Scholar] [PubMed]
- Poggi A, Zocchi MR. Immunomodulatory properties of mesenchymal stromal Cells: Still unresolved "Yin and Yang". Curr Stem Cell Res Ther 2019; 14: 344-350.
[Crossref] [Google Scholar] [PubMed]
- Jeong SG, Cho GW. Endogenous ROS levels are increased in replicative senescence in human bone marrow mesenchymal stromal cells. Biochem Biophys Res Commun 2015; 460: 971-976.
[Crossref] [Google Scholar] [PubMed]
- Luo J, Mills K, le Cessie S, Noordam R, van Heemst D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res Rev. 2020; 57: 100982.
[Crossref] [Google Scholar] [PubMed]
- Denu RA, Hematti P. Effects of oxidative stress on mesenchymal stem cell biology. Oxid Med Cell Longev 2016; 2016: 2989076.
[Crossref] [Google Scholar] [PubMed]
- Popova N, Ilchibaeva T, Naumenko V. Neurotrophic factors (BDNF and GDNF) and the serotonergic system of the brain. Biochemistry (Moscow) 2017; 82: 308-317.
[Crossref] [Google Scholar] [PubMed]
- Kuipers SD, Bramham CR. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: New insights and implications for therapy. Curr Opin Drug Discov Devel 2006; 9: 580-516.
[Google Scholar] [PubMed]
- Katila N, Bhurtel S, Shadfar S, Srivastav S, Neupane S, Ojha U, Jeong GS, Choi DY. Metformin lowers α-synuclein phosphorylation and upregulates neurotrophic factor in the MPTP mouse model of Parkinson's disease. Neuropharmacology 2017; 125: 396-407.
[Crossref] [Google Scholar] [PubMed]
- Azizi SA, Stokes D, Augelli BJ, di Girolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci U S A 1998; 95: 3908-3913.
[Crossref] [Google Scholar] [PubMed]
- Davis JM. Basic cell culture. Oxford University Press 2002.
- Dutta A, Bandyopadhyay S, Mandal C, Chatterjee M. Development of a modified MTT assay for screening antimonial resistantfield isolates of indian visceral leishmaniasis. Parasitol Int 2005; 54:119-122.
[Crossref] [Google Scholar] [PubMed]
- Nikoozad Z, Ghorbanian MT, Rezaei A. Comparison of the liver function and hepatic specific genes expression in cultured mesenchymal stem cells and hepatocytes. Iran J Basic Med Sci 2014; 17 (1), 27-33. [Crossref]
[Google Scholar] [PubMed]
- Sylvester PW. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol Biol. 2011; 716: 157-168.
[Crossref] [Google Scholar] [PubMed]