ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 20
Experimental study on the quantitative determination of ?-sitosterol in Fructus Bruceae extract and its inhibitory effect on gastric cancer metastasis
Chunlong Guo1, Xiaomin Zhu2* and Yueqiu Zhang1
1Department of Galactophore, Dalian Maternal and Child Health Care Hospital, Dalian Medical University Dalian Obstetrics and Gynecology Hospital, Dalian, Liaoning Province, PR China
2Department of Medicine Oncology, Dalian Municipal Central Hospital Affiliated of Dalian Medical University, Dalian, Liaoning Province, PR China
- *Corresponding Author:
- Xiaomin Zhu
Department of Medicine Oncology
Dalian Municipal Central Hospital Affiliated of Dalian Medical University, PR China
Accepted date: August 24, 2017
To establish an RP-HPLC method for the determination of β-sitosterol content in Fructus Bruceae extract; and to observe its inhibitory effect on gastric cancer metastasis. β-sitosterol content in Fructus Bruceae extract was determined by RP-HPLC. Inhibitory effects of low-, medium- and high-dose extracts on the adhesion of human gastric cancer SGC-7910 cells to Fibronectin (FN) were detected by MTT assay. Inhibitory effects of the extract on SGC-7910 cell invasion and migration were observed by reconstituted basement membrane invasion assay. Absorbance values of low-, medium- and high-dose Fructus Bruceae extract treatment groups were significantly different compared to the control group (P<0.01). After treatment with low-, medium- and high-dose extracts, the number of cells passing through the membrane differed significantly from the control group (P<0.05). After treatment with high-dose extract, the scratch area spacing was significantly different from the control group (P<0.01). Fructus Bruceae extract can inhibit the adhesion, invasion and migration of gastric cancer cells, which has an inhibitory effect on the gastric cancer metastasis. The RP-HPLC method established provides a reliable basis for the quantitative evaluation of Fructus Bruceae.
Keywords
Fructus Bruceae extract, β-sitosterol, RP-HPLC, Gastric cancer, Metastasis.
Introduction
Fructus Bruceae is the mature fruits of Brucea javanica (L.) Merr. in the genus Brucea of the family Simaroubaceae. Originally recorded in the "Supplement to Compendium of Materia Medica", it is also known as Laoyadan, Kushenzi, etc. The plant is bitter, cold and enters the large intestine and liver meridians. Fructus Bruceae has heat-clearing, detoxifying, malaria-preventing, dysentery-checking and wart-eliminating functions, which is used for bloody dysentery, hemafecia, malaria, corns, warts, etc. [1].
Gastric cancer is one of the most common malignancies in the world. According to statistics, there are 876,000 cases of newly diagnosed gastric cancer around the world each year, accounting for 9% of all new cases of cancer. About 647,000 people die of gastric cancer every year, which is the second leading cause of cancer deaths [2]. Domestic and foreign scholars have done extensive research on the anti-cancer effect of Fructus Bruceae and have fully proved its anti-cancer activity [3-5]. The primary cause of death from cancer is tumor invasion and metastasis. We observed the effects of Fructus Bruceae extract on the adhesion, invasion and migration of human gastric cancer SGC-7910 cells [6,7].
The currently reported methods for analyzing Fructus Bruceae include TLC scanning, GC, HPLC-UV detection, HPLCELSD, etc. [8,9]. In this study, the mass of Fructus Bruceae extract was controlled, and the β-sitosterol content in the extract was determined. Under the selected chromatographic conditions, β-sitosterol in the sample solution well separated from the adjacent components. Methodological evaluation showed that the reproducibility, stability, precision and sample recovery of the method were all in line with the relevant regulations. Thus, the method can be used for the mass control of Fructus Bruceae extract.
Materials and Methods
Instruments
Aglient 1200 HPLC system with G1315 DAD. BP-211D electronic balance (Sartorius); HS312D ultrasonic cleaner (Haitian Electronic Instrument Factory, Zhejiang). Thermo-3110 CO2 incubator; Multiskan FC microplate reader (Thermo Scientific). Reagents: Chloroform (Tianjin Shentai Chemical Reagents Co., Ltd.); anhydrous ethanol (Beijing Chemical Plant); methanol (chromatography pure, Tianjin Kemiou Chemical Reagent Co., Ltd.); phosphoric acid (Beijing Hongxing Chemical Plant). Β-sitosterol reference (National Institute for the Control of Pharmaceutical and Biological Products, Batch No. 11085-200504); Fructus Bruceae extract (self-prepared). MTT (Sigm); FBS culture, FN (GIBCO); Matrigel (Shanghai Bioleaf Biotechnology Co., Ltd.).
Cell line
Human gastric cancer SGC-7910 cells were purchased from the Nanjing Institute of Cell Biology (Batch No. 20160125sgc).
Methods
Preparation of Fructus Bruceae extract: Fructus Bruceae (600 g) medicinal material was crushed, soaked in a 3-fold amount of 95% ethanol overnight, then extracted under heat reflux for 2 h three times and filtered while hot. The filtrates were combined, concentrated under reduced pressure to a liquid and dried to give the Fructus Bruceae extract.
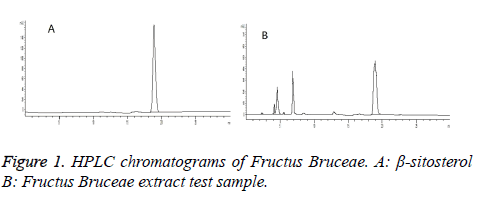
Chromatographic conditions: Column: Sinochrom ODS-BP (5 μm, 4.6 mm × 250 mm); detection wavelength: 210 nm; mobile phase: methanol-0.1% phosphoric acid (98: 2); and flow rate: 1.0 ml/min. Theoretical plate number was greater than 3,000. Chromatograms are shown in Figure 1.
Preparation of reference solution: 4.4 mg of β-sitosterol reference was accurately weighed, placed in a 10 ml volumetric flask, dissolved in anhydrous ethanol and diluted to the mark, then shaken well to give the reference solution.
Preparation of sample solution: About 5 g of Fructus Bruceae extract was accurately weighed, placed in a conical flask, soaked in 100 ml of chloroform for 1 h, then ultrasonicated (power 150 W, frequency 40 Hz) for 1 h and filtrated. After evaporation to dryness, the residue was dissolved in anhydrous ethanol, then transferred to a 25 ml volumetric flask, dissolved in anhydrous ethanol and diluted to the mark, shaken well and filtered through microporous membrane (0.45 μm) to give the sample solution.
Linearity investigation: 0.5, 1, 2, 5, 10 and 20 μL of the reference solution were accurately drawn and injected into the HPLC system for determination of peak area according to the above chromatographic conditions. Standard curve was plotted with peak area as the ordinate and reference mass as the abscissa to derive the regression equation: Y=29.637X+2.0813, r=0.9999. The results revealed good linearity within an injection amount range of 0.22-8.8 μg.
Precision test: 10 μL of reference solution was injected 5 repeated times under the above chromatographic conditions for separate determination of β-sitosterol peak area. RSD of β- sitosterol peak area was found to be 1.12%, indicating good precision of the instrument.
Stability test: Sample solution from the same sample was injected 0, 2, 4, 8 and 12 h after preparation, respectively, for determination. RSD of β-sitosterol content was calculated to be 1.95%, indicating the stability of the sample solution within 12 h.
Reproducibility test: Five aliquots of 50 mg of sample powder from the same batch were accurately weighed and prepared into sample solutions as per the method in the "Preparation of sample solution". Each 20 μL of the sample solutions were injected for determination separately. The results showed that the mean β-sitosterol content was 10.2%, with a RSD=2.10%.
Sample recovery test: Five aliquots of each 25 mg of sample powder were accurately weighed, added precisely with certain amounts of β-sitosterol reference and prepared as per the method in the "Preparation of sample solution", followed by determination of content and calculation of recovery. The results are listed in Table 1.
| Sample weight (mg) | Sample content (mg) | Reference addition (mg) | Measured total (%) | Recovery (%) | Average (%) | RSD (%) |
|---|---|---|---|---|---|---|
| 24.65 | 2.51 | 2.2 | 4.78 | 102.97 | 99.83 | 1.99 |
| 26.99 | 2.75 | 2.2 | 4.95 | 99.86 | ||
| 24.80 | 2.53 | 2.2 | 4.70 | 98.65 | ||
| 24.90 | 2.54 | 2.2 | 4.69 | 97.74 | ||
| 24.24 | 2.47 | 2.2 | 4.67 | 99.89 |
Table 1: Sample recovery test results.
Determination of sample content: Three different batches of Fructus Bruceae extract samples were prepared into sample solutions as per the method in the "Preparation of sample solution". Each 10 μL of the sample solutions was precisely drawn and determined according to the above chromatographic conditions. The results are listed in Table 2.
| Batch no. | ß-sitosterol (%) | RSD (%) |
|---|---|---|
| 1 | 10.52 | 2.12 |
| 2 | 10.84 | |
| 3 | 10.56 |
Table 2: Sample content determination results.
Preparation of drug containing sera: 40 wistar rats were randomly divided into the Fructus Bruceae extract low-, medium-, high-dose groups and control group. The rats in the low-, medium- and high-dose groups were intragastrically administrated with 200 mg/kg, 400 mg/kg and 800 mg/kg extract, while those in the control group were gavaged with the same volume of water. 1 week later, the rats were killed, and blood was sampled for serum separation.
Cell culturing: SGC-7910 cells were subcultured in a 37°C, 5% CO2 incubator with 10% FBS-containing RPMI-1640 medium for 48 h. The medium was replaced once every 24 h. After the bottom of culture flask was covered with SGC-7910 cells, the medium was discarded, and the cells were digested with 0.25% trypsin. The cell concentration was adjusted to 1 × 106/ml with the above medium.
Inhibitory effect of Fructus Bruceae extracts on adhesion of human gastric cancer SGC-7910 cells
1 × 106/ml gastric cancer SGC-7910 cells were seeded into FN-coated 96-well plates at 100 μl per well. Cells were divided into five groups: blank group, control group, Fructus Bruceae extract low-dose group, medium-dose group and high-dose group. Five replicate wells were set up for each group. Fructus Bruceae extract low-, medium-, high-dose groups were added with 100 μl of corresponding drug-containing sera, while the control group was not added with such. In the blank group, SGC-7910 cells were replaced with culture medium. The plates were incubated in a 37°C, 5% CO2, saturation humidity incubator for 90 min. After termination of treatment, the plates were washed twice with PBS solution, and absorbance of each well was measured by MTT colorimetric assay.
Inhibitory effect of Fructus Bruceae extract on invasion of human gastric cancer SGC-7910 cells
Transwell chambers were placed into the culture plates, with lower chambers each added with 1 ml of culture medium, and upper chambers added with 100 μl (1 × 106/ml) of SGC-7910 cell suspension. Methods of grouping and administration were the same as the adhesion experiment. After incubation in a 37°C, 5% CO2 incubator for 6 h, the Transwell chambers were removed, washed with PBS, wiped off of the cells on the upper of membrane with cotton swab, then fixed in 950 ml/L ethanol, and stained with 4 g/L trypan blue solution. Microscopically, the cells migrated to the lower layer of microporous membrane was counted.
Inhibitory effect of Fructus Bruceae extract on migration of human gastric cancer SGC-7910 cells
100 μl (1 × 106/ml) of SCG-7910 cells were seeded into FNcoated 96-well plates, grouped in the same manner as in the adhesion experiment and cultured routinely until formation of a cell monolayer. On the monolayer culture cells, linear scratches were made along the bottom of culture plates with pipette dropper, and the relative distance of scratch area was recorded under the microscope. Culturing was continued for an additional 24 h by adding Fructus Bruceae extract-containing sera at low-, medium- and high-doses, respectively, and then the migration distance was measured.
Statistical processing
All data were analysed using SPSS 12.0, and ANOVA was used for inter-group comparisons.
Results
Inhibitory effect of Fructus Bruceae extract on the adhesion of human gastric cancer SGC-7910 cells
Most of the cells in the control group were adherent, with morphology close to fusiform. A large number of cells treated with Fructus Bruceae extract-containing sera were nonadherent suspension cells. Moreover, a small number of adherent cells were still in the early stage morphology, presenting circular or irregular triangular shapes. MTT values of Fructus Bruceae extract low-, medium- and high-dose groups were significantly different from the control group (P<0.01). No significant difference was found though between the low-, medium-, high-dose groups (P>0.05). The results are listed in Table 3.
| Group | n | Absorbance |
|---|---|---|
| Control group | 5 | 366.45 ± 14.8 |
| Drug-containing sera low-dose group | 5 | 248.7 ± 19.6* |
| Drug-containing sera medium-dose group | 5 | 269.4 ± 18.2* |
| Drug-containing sera low-dose group | 5 | 258.5 ± 17.5* |
Table 3: Comparison of absorbance after cell adhesion between groups (x ± s).
Inhibitory effect of Fructus Bruceae extract on invasion of human gastric cancer SGC-7910 cells
Compared to the control group, the number of cells passing through membrane differed significantly for the Fructus Bruceae extract low-, medium- and high-dose groups (P<0.05), but no significant difference was found between the low-, medium-, high-dose groups (P>0.05, Table 4).
| Group | n | Number of cells |
|---|---|---|
| Control group | 5 | 207.5 ± 10.6 |
| Drug-containing sera low-dose group | 5 | 184.7 ± 9.5* |
| Drug-containing sera medium-dose group | 5 | 183.2 ± 11.3* |
| Drug-containing sera low-dose group | 5 | 179.2 ± 6.5* |
*Comparison with the control group, P<0.05
Table 4: Number of cells passing through membrane between groups (x ± s).
Inhibitory effect of Fructus Bruceae extract on migration of human gastric cancer SGC-7910 cells
Scratch area spacing was significantly different between the Fructus Bruceae extract high-dose group and the control group (P<0.01, Table 5).
| Group | n | Spacing (μm) |
|---|---|---|
| Control group | 5 | 219.2 ± 7.6 |
| Drug-containing sera low-dose group | 5 | 217 ± 9.5 |
| Drug-containing sera medium-dose group | 5 | 215.3 ± 6.2 |
| Drug-containing sera low-dose group | 5 | 207.2 ± 4.1* |
| *Comparison with the control group, P<0.01 | ||
Table 5: Scratch area spacing between groups (x ± s).
Discussion
Metastasis is one of the specific biological characteristics of malignancies, which is also the primary cause of death of patients with malignancies. About 80%-90% of gastric cancer metastasizes in the advanced stage, and more than half of patients die of metastasis and recurrence even if radical surgery is performed. Despite fairly extensive basic research on the metastasis of gastric cancer in recent years, effective approach for prevention and treatment of gastric cancer metastasis is still lacking clinically.
Traditional Chinese drugs have a certain advantage compared to chemical drugs because they have less toxicity and side effects. They provide a new channel for the development of non-toxic or low-toxic anticancer agents, which play an active role in the prevention and treatment of gastric cancer recurrence and metastasis [10,11]. Our previous study found that the Fructus Bruceae extract could inhibit the proliferation of human gastric cancer SGC-7910 cells and induce their apoptosis.
Tumor metastasis is a multi-factor, multi-step and multi-stage complex process. Malignant tumor invasion is done in three steps: in the first step, cells adhere to the Extracellular Matrices (ECMs); in the second step, tumor cells, by themselves or by inducing host cells, secrete proteolytic enzymes to degrade the matrices, thereby invading the surrounding tissues; and the last step is chemotaxis-mediated cell movement and migration [12].
During tumor cell metastasis, cellular adhesion to the extracellular matrix Laminin (LN) and Fibronectin (FN) is an early behavior of cell infiltration and metastasis. Adhesion is the prerequisite and basis of tumor cell metastasis. Heterogeneous adhesion between tumor cells and basement membrane is the premise for tumor cell invasion and metastasis, so inhibition of adhesion can further inhibit tumor invasion [13]. Their study used an artificial reconstituted basement membrane material fibronectin to simulate the extracellular basement membrane. The method allowed good observation of the adhesive ability of cancer cells to basement membrane. Results of the present study showed that the low-, medium- and high-dose Fructus Bruceae extract-containing sera significantly inhibited the adhesive ability of gastric cancer cells (P<0.01), and delayed the cancer cell attachment time. Tumor cell invasion refers to the degradation process of ECMs. Tumor cells adhere to the matrices or basement membrane LN, FN and type IV collagen via specific receptors on the membrane surface, then release the proteolytic enzymes or activate zymogen present in the matrices to degrade the matrix components, thereby invading the surrounding tissues. In this study, matrigel was deposited on the porous membrane of the Transwell invasion chamber, which can form a basement membrane structure highly similar to the natural one. Counting of cells entering the lower chamber can reflect the invasive ability of the cells. The results revealed significant differences in the number of cells passing through membrane after treatment with low-, medium- and high-doses of Fructus Bruceae extract-containing sera compared to the control group (P<0.05), indicating that the Fructus Bruceae extract can reduce the invasive ability of gastric cancer cells.
Tumor cells always accompany movement and migration during metastasis. When the tumor cells pass through the vascular or lymphatic basement membrane, they further move and migrate, and thereby invade the surrounding tissues. Therefore, cell movement is a prerequisite for invasion and metastasis [14]. The results of this study showed that 24 h after treatment of gastric cancer cells with Fructus Bruceae extractcontaining sera, the scratch area spacing of cells in the control group narrowed significantly, while that for the Fructus Bruceae extract high-dose group remained large. This indicates that the Fructus Bruceae extract can inhibit the movement of gastric cancer cells.
In-vitro observation of the effects of Fructus Bruceae extractcontaining sera on the adhesive, invasive and migratory abilities of gastric cancer SGC-7910 cells found that the Fructus Bruceae extract can inhibit the adhesion, invasion and migration of gastric cancer cells, with relatively strong adhesion inhibitory effect and relatively weak migration inhibitory effect. Adhesion, invasion, and migration are essential steps in tumor metastasis, which depend on and interact with each other to influence the entire process of tumor metastasis.
In this experiment, HPLC was employed to determine the β- sitosterol content in Fructus Bruceae extract. The operation was carried out at room temperature. The method was sensitive, simple and rapid, which can provide a reliable basis for the quantitative determination of β-sitosterol in Fructus Bruceae extract.
In the selection of extracting solvent, chloroform, methanol and ethanol were compared. The results showed that chloroform can not only achieve high extraction efficiency, but can also yield less impurities. In the selection of extracting method, Soxhlet extraction and ultrasonic extraction were compared. The results showed that the ultrasonication method had the advantages of short time, high efficiency and low cost, which was superior to the Soxhlet method.
When the mobile phase was methanol-water (98:2), the symmetry of peaks was poor. After replacing it with a methanol-0.1% phosphoric acid system, peak shape improved and the resolution efficiency was enhanced.
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People?s Republic of China Beijing Chemical Industry Press 2015; 1: 179-180.
- Mansour M, Izzo L, Mazzone G, Gabriele R, Cello PD, Basso L, Ranieri E, Costi U, Jovanovic T, Izzo P. Curative gastric resection for the elderly patients suffering from gastric cancer. G Chir 2016; 37: 13-18.
- Chen JH, Kim SH, Fan PW, Liu CY, Hsieh CH, Fang K. The aqueous extract of Chinese medicinal herb Brucea javanica suppresses the growth of human liver cancer and the derived stem-like cells by apoptosis. Drug Design Develop Ther 2016; 10: 2003-2013.
- Liu TT, Mu LQ, Dai W, Wang CB, Liu XY, Xiang DX. Preparation, characterization, and evaluation of antitumor effect of Brucea javanica oil cationic nanoemulsions. Int J Nanomedicine 2016; 11: 2515-2529.
- Anderson MM, ONeill MJ, Phillipson JD, Warhurst DC. In vitro cytotoxicity of a series of quassinoids from Brucea javanica fruits against KB cells. Planta Medica 1991; 57: 62-64.
- Hayao N, Kenzo Y, Yuzuru I, Hiroyuki Y, Seiichi M, Yasuhiro K, Masae T. Establishment and characterization of three novel human gastric cancer cell lines with differentiated intestinal phenotype derived from liver metastasis. Clin Exp Metastasis 2005; 22: 137-147.
- Park SS, Ryu JS, Min BW, Kim WB, Kim SJ, Kim CS, Mok YJ. Impact of skip metastasis in gastric cancer. ANZ J Surg 2005; 75: 645-649.
- Zhou CL, Chen XH, Bi KS. GC determination of oleic acid and linoleic acid in Brucea javanica (L.) Merr. Chinese J Pharm Anal 2006; 26: 996-998.
- Zhou Z, Shi R, Liu B, Zou J, Wang L, Xia J. Quantitative determination of contents of three components in Brucea javanica by HPLC. China J Chinese Materia Medica 2011; 36: 1979-1981.
- Yang JK, Zhen J, Shen KP. Clinical study on post-operative metastasis prevention of progressive gastric cancer by weichangan. CJITWM 2003; 23: 580-582.
- Xu YQ, Xue HN, Zhu XQ, Chen XD. Clincical observation on treatment for postoperative metastasis of gastric cancer by Jianpi Huoxue jiedu prescription. J Chin Integr Med 2003; 1: 192-194.
- Gao P, Xing AY, Zhou GY, Zhang TG, Zhang JP, Gao C, Li H, Shi DB. The molecular mechanism of microRNA-145 to suppress invasion-metastasis cascade in gastric cancer. Oncogene 2013; 32: 491-501.
- Schlaepfer DD, Mitra SK, Dic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta 2004; 1692: 77-102.
- Takanami I, Takeuchi K, Watanabe H, Yanagawa T, Takagishi K. Autocrine motility factor receptor gene expression and cell motility in lung cancer cell lines. Oncol Rep 2002; 9: 125-128.