ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 8
Expression of growth factors in re-epithelialization of diabetic foot ulcers after treatment with non-thermal plasma radiation
Department of Medical Sciences, Ardabil Branch, Islamic Azad University, Ardabil, Iran
- *Corresponding Author:
- Mohammad Zaefizadeh
Department of Medical Sciences, Ardabil Branch
Islamic Azad University
Ardabil, Iran
Accepted date: November 09, 2016
Background: Amputation in diabetic patients is a very important risk factor in reducing quality of life and one of the most costly complications of diabetes. Studies have shown that non-thermal plasma effects on wound healing are very promising and have no or minimal impact on the surrounding tissues. Therefore, in this study we investigated the efficiency of plasma jet therapy in treatment of wounds and the mechanism of its effects on proteins.
Methods: In this study we tested plasma radiation on four patients with diabetic foot ulcers. Plasma radiation was performed on three patients with the fourth as a negative control. Biopsy samples were taken from the patients’ wounds before and after plasma radiation and differentially-expressed proteins were identified using two-dimensional electrophoresis and matrix-assisted laser desorption ionization - time of flight - time of flight (MALDI-TOF-TOF).
Results: All three patients had improved wounds after plasma radiation and the results of mass spectrometry of differential proteins indicated up-regulation of keratinocyte growth factor (KGF), transforming growth factor beta-1 (TGFb-1) and epidermal growth factor (EGF) in the healing ulcers.
Conclusion: Since previous studies have shown that growth factors play an important role in many processes such as tissue repair, re-epithelialization of the skin and stimulation of cell proliferation and migration, an increase in these proteins after plasma radiation is likely to be responsible for the beneficial effect of plasma radiation on wound healing.
Keywords
Growth factors, Diabetic foot ulcers, Proteomics, Plasma jet.
Introduction
Diabetes mellitus is the name for a group of metabolic diseases characterized by hyperglycaemia resulting from defects in insulin secretion, insulin action, or both [1]. Diabetes is becoming one of the most serious and most important health problems in human and economic terms, and is one of the costliest diseases worldwide [2]. Its prevalence has increased in recent decades due to population growth, aging, urbanization, obesity and physical inactivity [3]. International Diabetes Federation statistics show that, in 2014, 3.8% of adults suffered from diabetes, which represents approximately 387 million people worldwide. It is expected that by 2035 this number will increase by 53%, to 592 million people [4]. The chronic hyperglycaemia of diabetes is associated with longterm damage, dysfunction, and failure of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels [5]. In the meantime, one of the most common complications of diabetes is diabetic foot ulcers, which occur due to abnormalities of nerves and vessels [6]. Investigations suggest that the prevalence of complications of diabetic foot ulcers is between 5.3 and 10.5%, with over 25% of diabetic patients being affected by a diabetic foot ulcer during their lifetime [7]. Studies have shown that only two-thirds of these patients eventually recover and up to 28% of these cases lead to amputation [8]. Although various improvements in the treatment of diabetic foot ulcers have been achieved, they are complementary to each other and there is a need for more effective treatments. The disadvantages of existing treatments include the high cost of treatment, long-term hospitalization, damage to healthy tissue, inefficient treatment of infections and even death [9]. On the other hand, considering the effects and various functions of non-thermal plasma-an almost ionized gas stream which is obtained under atmospheric pressure and a temperature close to the environment-in various branches of engineering as well as in medicine, and in light of the results of non-thermal plasma effects on skin diseases [10,11] and wounds and the healing properties of epithelial cells in the skin, it has been suggested that plasma jets could be useful candidates for therapy [12]. Diabetes and risk factors of diabetic foot ulcers are multifactorial diseases. Consequently, changes in protein profiles and identification of proteins effective in the process of healing is certainly considered important in increasing treatment efficiency. We carried out this study to perform effective protein profiling and to investigate changes in protein expression with treatment.
Materials and Methods
Sample selection
After obtaining permission from the university ethics committee, four patients with type 2 diabetes who had diabetic foot ulcers were recruited. Exclusion criteria in this study included people aged less than 18 years, pregnant women, people with cancer or dementia. It was explained to the patients how the results will be used and their informed consent was obtained.
Plasma jet radiation
Plasma radiation was performed in seven sessions over 15 days (every other day), for one minute each time. The treatment was administered to three patients and the fourth patient was left untreated (negative control). Biopsy samples were taken from all the patients’ wounds before and after plasma radiation. Figure 1 shows the plasma jet.
Protein extraction and two-dimensional electrophoresis
Each sample was crushed in a mortar cooled with liquid nitrogen. Then, 2 mL of protein extraction buffer was added; the composition of the buffer is shown in Table 1. After being thoroughly homogenized, the resulting solution was transferred into 2 mL vials and centrifuged for 10 min at 8,000 RPM. Then, the supernatant was transferred into another clean vial and protein concentration was measured by the Bradford method.
| Tris-Hcl (PH=7.4) | 3.93 g | 50 mM |
|---|---|---|
| Urea 6M | 180 g | 6 M |
| β-Mercaptoetanol (2.5%) | 12.5 mL | 2.50% |
| SDS | 10 g | 2% |
Table 1. Composition of protein extraction buffer.
Equal amounts of protein were used for two-dimensional electrophoresis with an Immobilize PH Gradient (IPG), using strips with a length of 18 cm and a pH in the range of 10-3. After electrophoresis in the first and second dimensions, the gels were stained using Coomassie blue, and after decolorization and appearance of protein spots, the gel was scanned and images were stored. Protein patterns were analyzed using Melanie software, Version 7, and proteins (spots) were detected and categorized with regard to their presence or absence and whether they were up-regulated or down-regulated. The differential spots were confirmed in the replicates and control and were selected for identification of the proteins. For identification of spots using mass analysis by Matrix Assisted Laser Desorption Ionization-Time of Flight- Time of Flight (MALDI-TOF-TOF), the samples were separated from the gel and sent to York University, UK.
Results
Results of plasma radiation
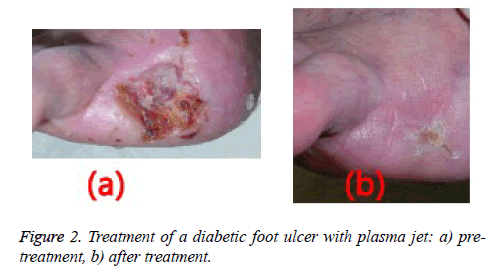
Wound healing was observed following one week of plasma radiation to three diabetic ulcers in separate patients (Figure 2). This finding was consistent with the results of tests on mice with wounds treated by non-thermal plasma radiation for 10 seconds the wounds coagulated quickly and healed well [13].
Results of two-dimensional electrophoresis
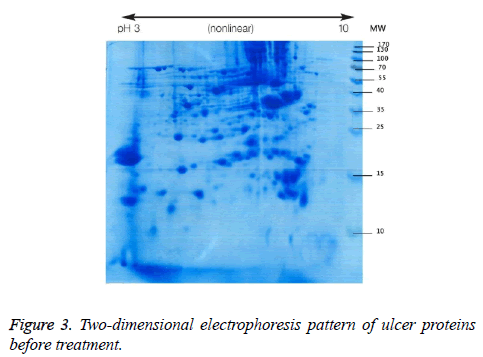
We used two-dimensional electrophoresis to evaluate protein expression in wound biopsy tissue. The gels obtained from samples taken before and after radiation treatment is shown (Figures 3 and 4).
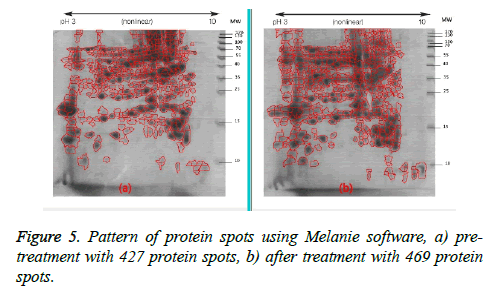
Following two-dimensional electrophoresis the protein pattern revealed the presence of 427 spots in the gel before treatment and 469 in the gel after treatment (Figure 5). This pattern did not differ from that of the negative control samples.
For identification purposes, using mass analysis by MALDI TOF-TOF and enzyme treatment with trypsin, three spots were selected. These showed the greatest difference from other spots.
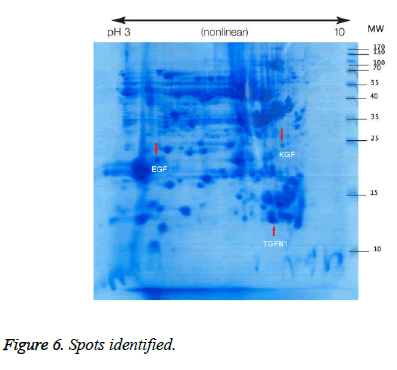
Spot 1: Transforming growth factor beta-1
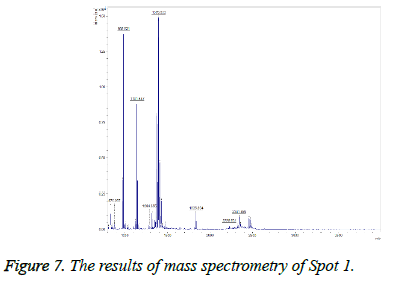
This spot was not present in the gel before treatment, but was observed after treatment (Figure 6), and on the gel it was found in the position of MW=13 and alkaline pH and TGFb1 proteins were identified by MALDI TOF-TOF. The results of mass spectrometry (Figure 7) revealed this spot, with a Mascot score of 218, exhibited 51% overlap with transforming growth factor beta protein. The error rate (E value) calculated in identifying this protein from the generated fragments is equal to 0.000016.
Spot 2: Epidermal growth factor
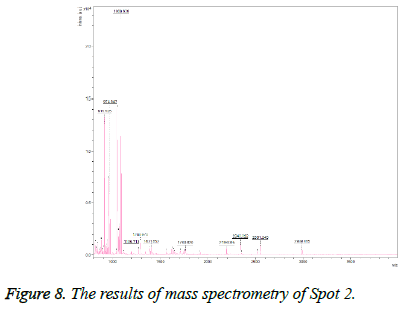
This spot was also not present in the gel before treatment, but was observed after treatment and was identified as epidermal growth factor (Figure 6). On the gel, it was found in the MW=25 position and acidic pH. The results of mass spectrometry (Figure 8) revealed that this spot, with a Mascot score of 370 and 58% overlap with epidermal growth factor protein, has an E value of 0.0000097.
Spot 3: Keratinocyte growth factor
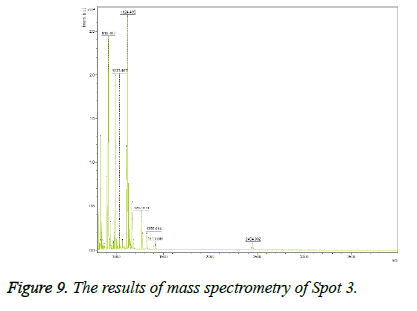
Keratinocyte growth factor protein was detected with 34% overlap, E value=0.0000012 and Mascot score=350 and its expression was upregulated on the gel after treatment. This spot on the gel was placed in the position of MW=22 and alkaline pH. The results of mass spectrometry of this spot are shown in Figure 9.
Discussion and Conclusion
Wound healing is a complex physiological process and in diabetic patients is known to be affected by many factors [14,15]. One of these factors is a disturbance in the production of growth factors [16]. Wound healing occurs as a cellular response to damage and includes activation of keratinocytes, fibroblasts, endothelial cells, macrophages and platelets [17].
Spot 1 (TGF-β1) and spot 2 (EGF) were not detected in the electrophoresis gel before treatment, but after treatment they were both observed while spot 3 (KGF) was upregulated on the gel after treatment. This increase therefore appeared to be related to the plasma treatment.
TGF-β was first identified in 1983 and is one of the cytokine complexes with extensive effects on growth and development which also plays a role in wound healing [18,19]. TGF-β is produced by macrophages, fibroblasts, keratinocytes, lymphocytes, and active platelets [20]. TGF-β1, TGF-β2 and TGF-β3 are the main forms in mammals, but it is predominantly TGF-β1 which is involved in wound healing [21]. This growth factor is a versatile polypeptide that has effects on the proliferation and differentiation of cells. It also functions in stimulating chemotaxis and angiogenesis [22]. Another factor is EGF, which was first identified in 1962 in the salivary glands of mice and is secreted by platelets, macrophages, monocytes and fibroblasts that work in both an autocrine and paracrine manner in epidermal cells, smooth muscle cells and fibroblasts. Its primary role is to stimulate the growth of epithelial cells around the wound [23,24].
Brown et al. reported in 1991 that EGF stimulates the division and migration of epidermal and stromal cells and promotes angiogenesis, and it is a powerful mitogen for keratinocytes [25]. As Tiaka et al. said the treatment of diabetic foot ulcers is fairly difficult work [24]. They noted that epidermal growth factor plays an important role due to its effect on epithelial cells and fibroblasts in repair of damaged epithelium [24]. In addition, Hong et al. determined the efficacy of recombinant epidermal growth factor in healing of diabetic foot ulcers and demonstrated that local treatment with EGF may have positive effects [26].
Keratinocyte growth factor (KGF) is a member of the FGF family, also called FGF7; this factor is produced by fibroblastic cells, and was discovered in 1989. It plays an important role in many biological processes including embryonic development, morphogenesis, cell growth and tissue repair [27,28]. Bennett et al. reported in 2003 that during the reconstruction of healthy skin epithelium, the expression of KGF increased and caused cell proliferation and migration [29]. KGF probably organizes the stimulation of dermal and epidermal signals to reepithelialize the wound and is important in the treatment of diabetic foot ulcers where re-epithelialization of the wound does not occur [29]. As Nakajima et al. described in 2014, although the mechanism of interaction between cold plasma and cells or living tissue is not clear, several studies have reported beneficial effects of cold plasma on key cells associated with wound healing, and re-integration of epithelium and wound are affected by the presence of growth factors such as EGF, KGF and TGF-β [30]. In addition, Fitzmaurice et al. stated in 2011 that non-thermal plasma free radicals can increase the phagocytosis of pathogenic bacteria, improving the wound-healing process and causing vasodilatation, vascular growth and secretion of cytokines (VEGF, bFGF and TGF-β) [31]. Free radicals increase the proliferation of fibroblasts and keratinocytes, induce collagen synthesis, and play an important role in antibacterial activity as well as increasing levels of growth factors and cytokines which are involved in signaling pathways [31].
In this study, the wounds of all three patients with diabetic foot ulcers were improved after plasma jet radiation. This result is consistent with those of Friedman et al. [10] and Ghoranneviss et al. in 2011 who reported that non-thermal plasma did not damage the tissues and that its use led to skin stimulation and reconstruction [13]. Isbary et al. [32] and Alkawareek et al. [33] showed that non-thermal plasma improves wound healing by reducing wounds’ bacterial load. Daeschlein et al. [34] used cold plasma to treat bacterial infections and stated that Staphylococcus and Lotus Micrococcus bacteria were destroyed. In 2014, Nakajima et al. [30] reported the effect of cold plasma in the development of fibroblasts and endothelial cells. Weltmann et al. stated in 2010 that treatment with plasma is promising for chronic wounds, skin diseases and tissue engineering or tumor treatment [35].
Kolb et al. reported in 2008 that all the yeast in agar medium was eradicated after a few seconds of plasma treatment, while cold plasma radiation even at 10 times more than the required amount to eliminate pathogens on the skin of laboratory animals did not cause any tissue damage [36]. Heinlin et al. also reported in 2010 the effectiveness of plasma in wound healing, tissue regeneration and treatment of infected chronic wounds and skin infections [37].
Generally, it can be concluded from this study that non-thermal plasma has therapeutic effects on diabetic ulcers, and plasma radiation seems to act by reducing the wound’s bacterial load, thus reducing infection in chronic wounds, causing proliferation of fibroblasts and endothelial cells and also increasing levels of growth factors.
This study is limited by the small number of patients and control samples and the small number of identifying spots and it is suggested that in order to verify these results and identify other effective factors a study with a larger sample size will be needed.
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37: S81-S90.
- Pan C. Diabetes care in China: meeting the challenge. World Hosp Health Serv 2005; 41: 29-30, 32.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047-1053.
- http://www. Idf.org/diabetesatlas
- American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care 2014; 37 Suppl 1: S14-80.
- Singh S, Pai DR, Yuhhui C. Diabetic foot ulcer–diagnosis and management. Clin Res Foot Ankle 2013; 1: 1-9.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005; 293: 217-228.
- Cheer K, Shearman C, Hude EB. Managing complications of the diabetic foot. BMJ 2009; 339: 1304-1307.
- Keshavarzi A, Larijani B, Tehrani M. Modern treatment of diabetic foot ulcers. J Med Coun Islam Rep Iran 2012; 29: 376-390.
- Fridman GR, Friedman GA, Gutsol A, Shekhter AB, Vasilets VN, Fridman A. Applied Plasma Medicine. Plasma Process Polym 2008; 5: 503-533.
- Tuhvatulin AI, Sysolyatina EV, Scheblyakov DV, Logunov DY, Vasiliev MM, Yurova, MA, Ermolaeva SA. Non-thermal plasma causes p53-dependent apoptosis in human colon carcinoma cells. Acta naturae 2012; 4: 82.
- Ghoranneviss M, Mohammadi S. Industrial and medical application of plasma jet INT. Workshop on Plasma Science and Applications, Thailand, 2012; 11-14.
- Ghoranneviss M, Janani E. Blood coagulation by low energy plasma jet INT. Symposium on plasma Chemistry 2011; 1-3.
- Rai NK, Tripathi K, Sharma D, Shukla VK. Apoptosis: a basic physiologic process in wound healing. Int J Low Extrem Wounds 2005; 4: 138-144.
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003; 83: 835-870.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005; 366: 1736-1743.
- Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007; 117: 1219-1222.
- Frolik CA, Dart LL, Meyers CA, Smith DM, Sporn MB. Purification and initial characterization of a type beta transforming growth factor from human placenta. Proc Nat Acad Sci 1983; 80: 3676-3680.
- Klass BR, Grobbelaar AO, Rolfe KJ. Transforming growth factor ß1 signalling, wound healing and repair: a multifunctional cytokine with clinical implications for wound repair, a delicate balance. Postgrad Med J 2009; 85: 9-14.
- Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med 1987; 165: 251-256.
- O'Kane S, Ferguson MW. Transforming growth factor beta s and wound healing. Int J Biochem Cell Biol 1997; 29: 63-78.
- Pertovaara L, Kaipainen A, Mustonen T, Orpana A, Ferrara N, Saksela O, Alitalo K. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem 1994; 269: 6271-6274.
- Cohen S. The stimulation of epidermal proliferation by a specific protein (EGF). Dev Biol 1965; 12: 394-407.
- Tiaka EK, Papanas N, Manolakis AC, Georgiadis GS. Epidermal growth factor in the treatment of diabetic foot ulcers: an update. Perspect Vasc Surg Endovasc Ther 2012; 24: 37-44.
- Brown GL, Curtsinger L, Jurkiewicz MJ, Nahai F, Schultz G. Stimulation of healing of chronic wounds by epidermal growth factor. Plast Reconstr Surg 1991; 88: 189-194.
- Hong JP, Jung HD, Kim YW. Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. Ann Plast Surg 2006; 56: 394-398.
- Werner S. Keratinocyte growth factor: a unique player in epithelial repair processes. Cyt & Growth Fact Rev 1998; 9: 153-165.
- Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Nat Acad Sci 1989; 86: 802-806.
- Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg 2003; 90: 133-146.
- Nakajima Y, Mukai K, Rahayu HSE, Nur M, Ishijima T, Enomoto H, Uesugi Y, Sugama J, Nakatani T. Cold plasma on full-thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialization and wound contraction. Clin Plas Med 2014; 2: 28-35.
- Fitzmaurice SD, Sivamani RK, Isseroff RR. Antioxidant therapies for wound healing: a clinical guide to currently commercially available products. Skin Pharm Physiol 2011; 24: 113-126.
- Isbary G, Morfill G, Schmidt HU, Georgi M, Ramrath K, Heinlin J, Karrer S, Landthaler M, Shimizu T, Steffes B, Bunk W, Monetti R, Zimmermann JL, Pompl R, Stolz W. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Bri J Dermatol 2010; 163: 78-82.
- Alkawareek MY, Gorman SP, Graham WG, Gilmore BF. Potential cellular targets and antibacterial efficacy of atmospheric pressure non-thermal plasma. Int J Antimicrob agents 2014; 43: 154-160.
- Daeschlein G, Scholz S, Ahmed R, Von Woedtke T, Haase H, Niggemeier M, Kindel E, Weltmann KD, Juenger M. Skin decontamination by low-temperature atmospheric pressure plasma jet and dielectric barrier discharge plasma. J Hosp Infect 2012; 81: 177-183.
- Weltmann KD, Kindel E, von Woedtke T, Hähnel M, Stieber M, Brandenburg R. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure Appl Chem 2010; 82: 1223-1237.
- Kolb JF, Mohamed AAH, Price RO, Swanson RJ, Bowman A, Chiavarini RL, Stacey M, Schoenbach KH. Cold atmospheric pressure air plasma jet for medical applications. Appl Phys Lett 2008; 92: 241501.
- Heinlin J, Morfill G, Landthaler M, Stolz W, Isbary G, Zimmermann JL, Shimizu T, Karrer S. Plasma medicine: possible applications in dermatology. JDDG 2010; 8: 968-976.