ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 3
Expression of myogenin and CD105 in autologous bone marrow derived mesenchymal stromal stem cells of Duchenne muscular dystrophy patients.
Sumit Jhajharia1*, Tapaswini Pradhan1 Ashok Kumar Das1, Prakash C.Mohapatra2
1Department of Biochemistry, Kalinga Institute of Medical Sciences, Bhubaneswar, Odisha , India.
2Principal, S.C.B Medical College, Cuttack, Odisha, India
- Corresponding Author:
- Sumit Jhajharia
Department of Biochemistry
Kalinga Institute of Medical Sciences
Bhubaneswar 751024, Odisha , India
Accepted Date: April 04 2015
Duchenne muscular dystrophy (DMD) is a severe recessive X-linked form of muscular dystrophy caused by mutations in the DMD gene located on the X chromosome,(Xp21). It is characterized by rapid progression of muscle degeneration, eventually leading to loss of ambulation and death. Dystrophin is the predominant DMD transcript expressed in striated muscle. DMD gene mutations, deletions or duplications most frequently result in loss of dystrophin expression in the muscle of these patients. Deletions are most commonly located in the 5' end and in the central region of the exons 44 to 52, of the gene. Application of Bone marrow derived mesenchymal stem cells represent a promising approach for supporting new clinical concepts of cell based therapy in regenerative medicine. The non hematopoietic component of bone marrow includes multipotent mesenchymal stem cells capable of differentiating into fat, bone, muscle, cartilage and endothelium. The present study was undertaken to assess the expression of Myogenin and CD105 in autologous bone marrow derived mesenchymal stromal cells of Duchenne Muscular Dystrophy patients. Ten DMD patients between 12 yrs to 24 yrs and 10 healthy controls were enrolled. With informed consent the bone marrow mononuclear cells were harvested, cultured and assessed for the expression of Myogenin and CD105 using Flourescence Activated Cell Sorter (FACS analysis). A significant increase in expression of Myogenin and CD105 was observed in the cultured autologous bone marrow derived Mesenchymal stromal cells of Duchenne Muscular Dystrophy patients. The expression of the above mesenchymal stem cell markers in patients with Duchenne Muscular Dystrophy indicate that autologous transplantation of bone marrow derived mesenchymal stromal cells could be of therapeutic potential.
Keywords
Mesenchymal stromal cells(MSCs), Duchenne Muscular Dytrophy(DMD), Myogenin, CD105, Flourescence Activated Cell Sorter (FACS)
Introduction
Duchenne muscular dystrophy (DMD) is a severe recessive X-linked form of muscular dystrophy[1-3] characterized by rapid progression of muscle degeneration, eventually leading to loss of ambulation and death having an incidence of 1 in 3,500[4] newborn males.
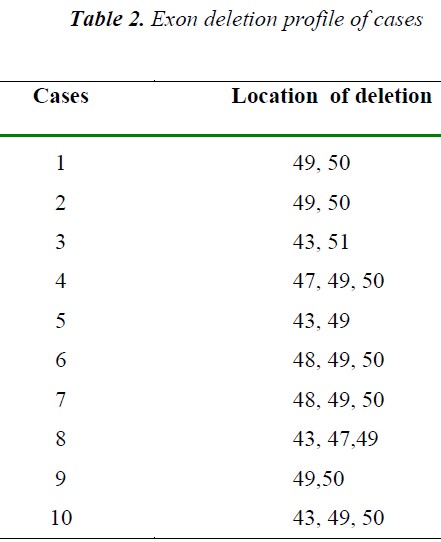
DMD is caused by mutations in the DMD gene, which is located on the X chromosome.(Xp21)[5]. Dystrophin is the predominant DMD transcript expressed in striated muscle. DMD gene mutations, deletions or duplications [6] most frequently result in a loss of dystrophin expression in muscle of these patients. Deletions are most commonly located in the 5' end and in the central region of exons 44 to 52, of the gene.[7]
In DMD muscle because of lack of dystrophin, the Dystrophin associated Glycoprotein Complex (DGC) does not form in the sarcolemma. In a complex cascading process involving several pathways, increase in oxidative stress within the cell damages the sarcolemma, and eventually results in death of the cell. Muscle fibers undergo necrosis and are ultimately replaced with adipose and connective tissue.
The only pharmacological therapy shown to benefit DMD patients is corticosteroid treatment,[8] which is associated with significant side-effects. Promising research is being conducted to find a therapy able to mitigate the detrimental effects of this affliction. There are many avenues currently under intense investigation, including stem cell replacement therapy,[9] exon skipping [10] analog up-regulation, and gene replacement.
Multipotent stem cell, termed pericytes [11] are located within the blood vessels of skeletal muscle. These cells can be delivered systemically and be taken up by crossing the vascular barrier. Once past the vasculature, pericytes have the ability to fuse and form myotubes. Therefore they can be injected arterially, cross through arterial walls into muscle, where they can differentiate into potentially functional muscle.
Mesenchymal Stem Cells (MSCs) have the ability to engraft into muscle tissue after systemic delivery. MSCs delivered intra-arterially can “home” to the site of injury[12-14] using “homing signal,” such as Stromal cell Derived Factor-1 (SDF-1) or Monocyte Chemotactic Protein-3 (MCP-3), and surface expression of C-X-C Chemokine Receptor 4 (CXCR4) receptors on MSCs. [15,16]
Immunomodulatory properties of MSCs [17,18] have shown to have implications in the treatment of inflammation indicating that immunosuppression is not always necessary after MSCs transplantation. Furthermore, an “immune phenotype” of MSCs has been described by other cell markers: MHC I+, MHC II”, CD40", CD80", and CD44+, CD73+, CD90+, CD105+.
These findings show a potential for stem cell therapy of DMD. Hence the present study was undertaken to study the differentiation potential of MSC by looking for the expression of skeletal muscle markers, myogenin and CD 105 in autologous bone marrow derived mesenchymal stromal stem cells in duchenne muscular dystrophy patients.
Material and Methods
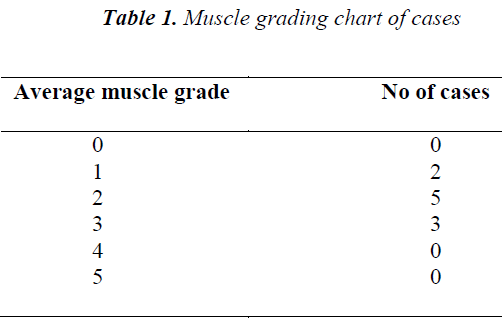
The present study was carried out in the Department of Biochemistry, S.C.B Medical College, Cuttack. A total of 10 clinically confirmed DMD cases based on high creatine kinase (1977± 147.43), muscle grade
Separation of mononuclear cells
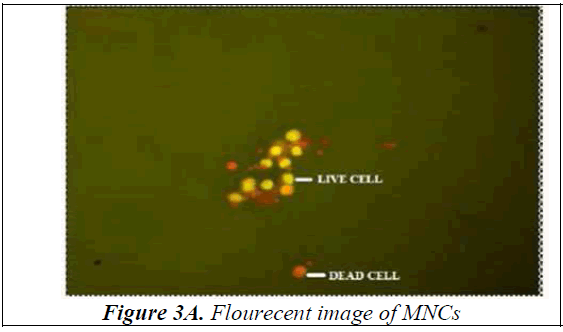
Bone Marrow was collected from the Anterior Superior Iliac Spine or Sternum under local anaesthesia. Mononuclear cells were separated using Ficoll- Paque. The viability of isolated mono-nuclear cells was done using Acridine Orange. The cells were observed under the fluorescent microscope and pictorially documented (Figure 3A, 3B) The viability of the mono-nuclear cells isolated was in the range of 90-95%.
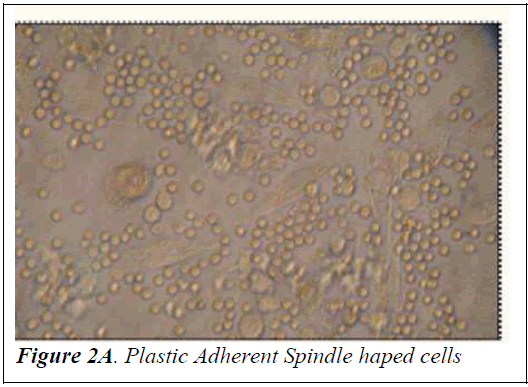
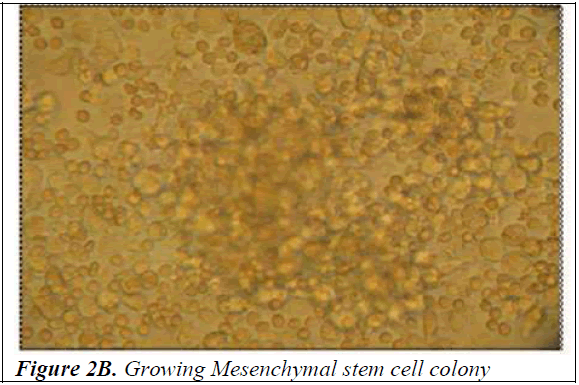
Culture of mononuclear cells The Mononuclear cells were washed with sterile Phosphate Buffered Saline (PBS) and then plated on gelatin coated T-25 filtered capped Nunc flasks in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose (Himedia) supplemented with 10% fetal bovine serum (FBS) (PAA laboratories GmbH, Australia) and 1% penicillin-streptomycin (Himedia). The culture flasks were incubated at 37°C with 5% carbon dioxide for initial 4 weeks prior to trypsinizing for further analysis. Half of the media was replaced once every 3-4 days. After incubation for a fortnight, spindle shaped cells appeared (Figure 2A). At the end of 4 weeks the cells were trypsinized using 0.25% trypsin-0.2% EDTA and tested for the presence of mycoplasma using the Mycoplasma mycoalert detection kit (Cambrex Bio Science, Baltimore, MD) to detect any contamination. Photographs of mononuclear cells during the short term culture were taken with Leica Photomicroscope (Figure 2B).
Characterisation of mesenchymal stem cell staining. The cell surface antigen expression of cultured cells was used for the characterization of mesenchymal stem cell phenotype as well as to evaluate the property of the cells to differentiate into the myogenic lineage. To stain for cell surface antigens (CD105 and CD34), the cells were suspended in FACS staining buffer and stained with anti-human CD105-APC (Biolegend) and antihuman CD34- FITC (Biolegend) separately. To stain for intracellular proteins (MyoD and Myogenin) the cells were resuspended in fixation buffer for 20mins at room temperature after washing with PBS. They were then washed and incubated with anti-human MyoD, anti-human Myogenin and Mouse IgG (Isotype) for 30mins. Again the cells were washed and stained for cells surface and intracellular antigens and analysed using FACS. The intracellular stained cells were then incubated with anti-mouse secondary antibody tagged with FITC (Jackson immunologicals, USA) at 1:50 dilution with permeabilization buffer. These cells were then incubated with the secondary antibody for 30mins at room temperature before washing again. Finally they were suspended in FACS buffer prior to taking them for FACS analysis in FACS Caliber (BD Biosciences).
Instained cells and cells stained with isotype were taken as negative control for analysis.
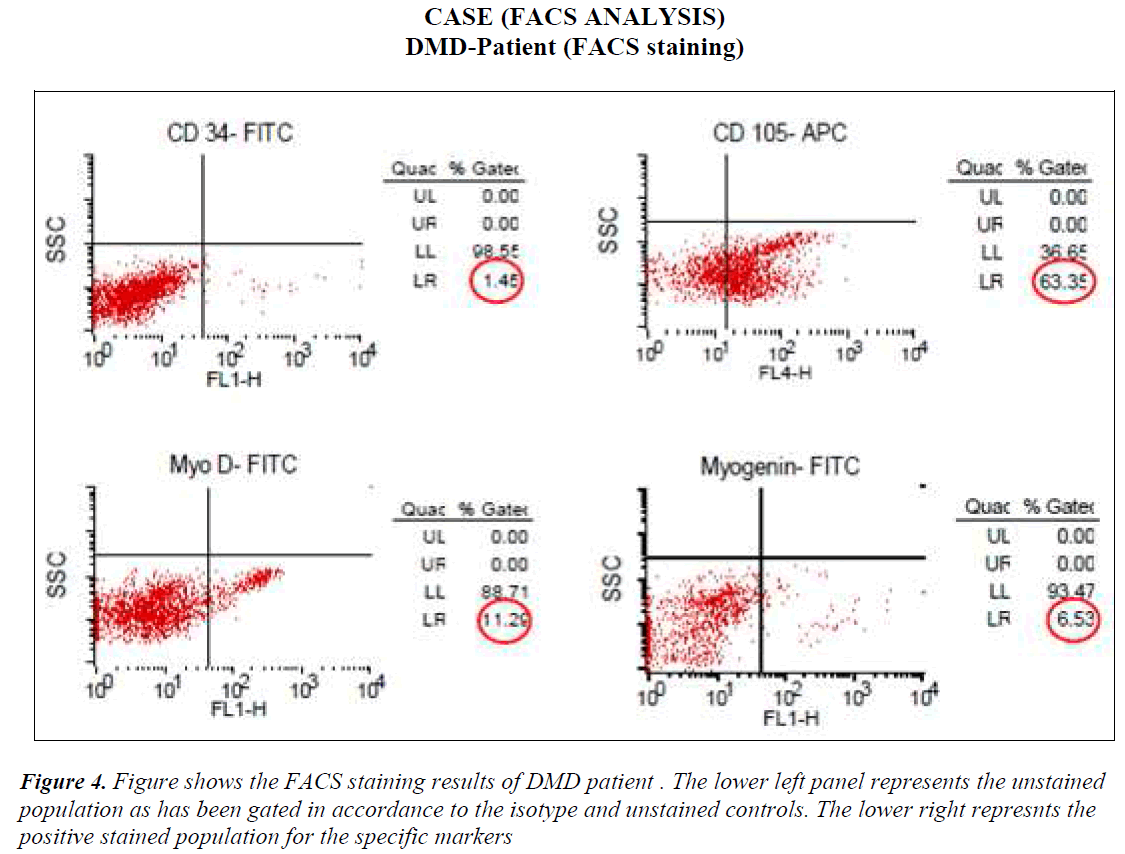
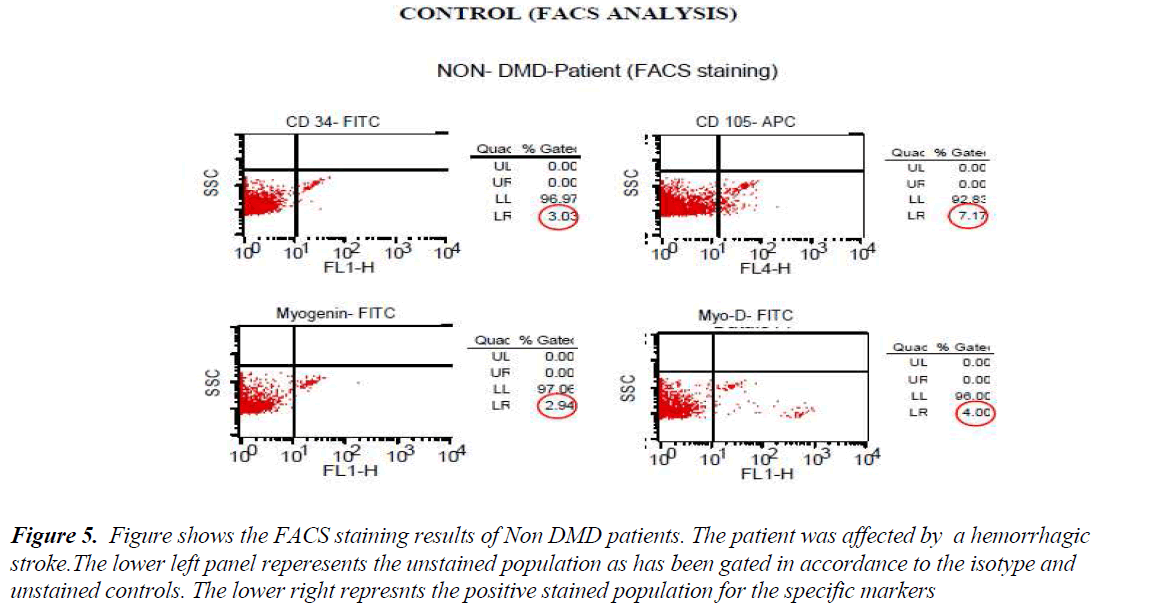
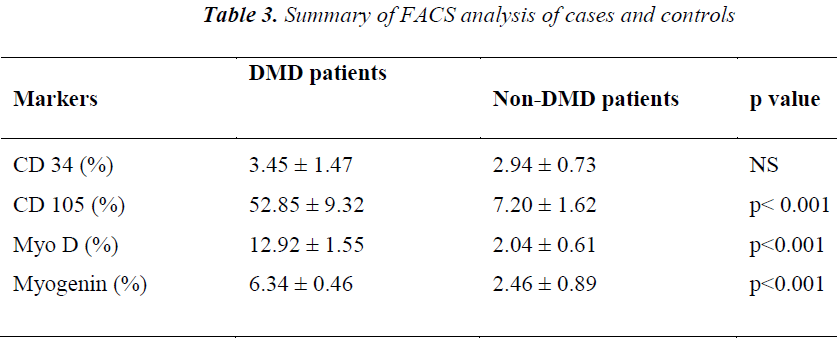
FACS analysis The unstained cells were first analysed with Dot plot graphical representation. Then isotype stained cells followed by test sample cells were analysed without altering the gating for unstained and/or isotype controls. All the cells except the ones in the gate of unstained or isotype cells were taken as positive staining. The values of all positive stained cells for cases (DMD) and controls (Non-DMD ) were recorded (Figures 4 and 5).
Figure 5: Figure shows the FACS staining results of Non DMD patients. The patient was affected by a hemorrhagic stroke.The lower left panel reperesents the unstained population as has been gated in accordance to the isotype and unstained controls. The lower right represnts the positive stained population for the specific markers
Statistical analysis Statistical significance was calculated using two-tailed student T-test.
Results
As shown in Table III, analysis of the stained cells by FACS revealed more number of cells positive for mesenchymal lineage (CD 105) in the bone marrow cells isolated from DMD patients, as compared to Non-DMD cases. In DMD patients, the mean CD105 count was 52.85 ± 9.32 % in comparison to Non- DMD patients where the count was 7.2 ± 1.62 % (p value < 0.001). There was a non significant difference in CD 34 cell count between DMD patients and Non-DMD cases. The significant difference in CD105+ population in DMD and Non-DMD cases was suggestive of the capacity of the mono-nuclear cells in preferentially differentiating into myogenic lineage in comparison to haematopoietic lineage.
In order to further investigate the property of the mesenchymal stem cell population, they were stained with muscle specific markers, such as MyoD, an early muscle specific transcription factor and Myogenin, a late muscle specific marker. The results revealed significantly more cells positive for MyoD (12.92 ± 1.55 %) and Myogenin (6.34 ± 0.46 %) in mononuclear cells isolate from DMD patient as compared to MyoD (2.04 ± 0.61 %) and Myogenin (2.46 ± 0.89 %) in that of Non-DMD controls(pvalue<0.001).
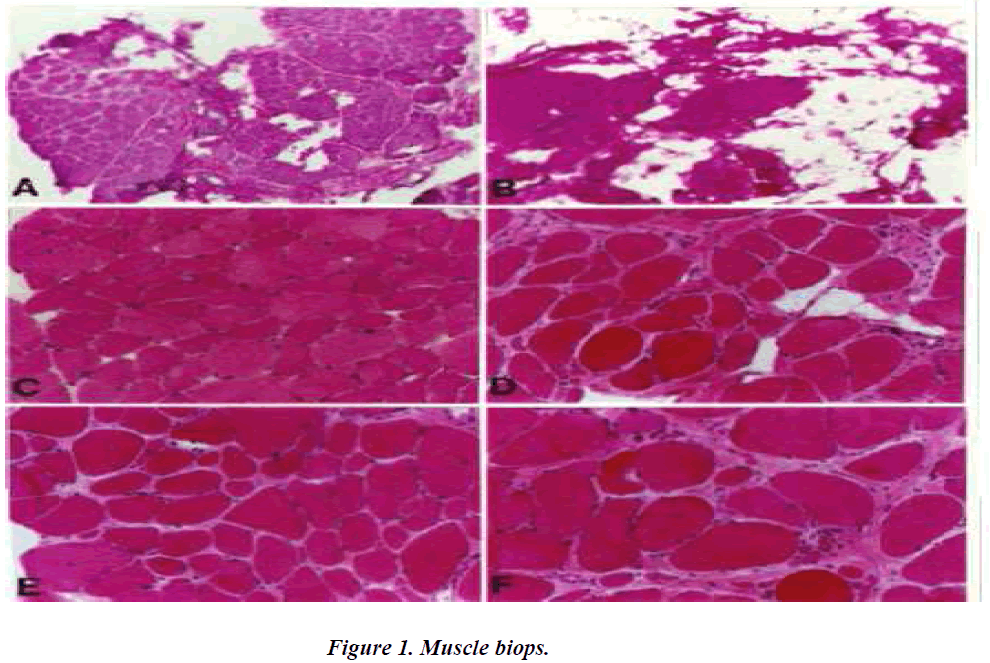
A.C.E. Three dystrophin-positive muscle biopsy specimen exhibit less dystrophic characteristics than the contralateral dystrophin-negative biopsy specimen (B.D.F.). Dystrphic characteristics include increases in fat and connective tissue, fibre spilitting, central nucleation, round and oval fibres.
Discussion
Results of the above study indicate an underlying signalling mechanism that triggers the myogenic regenerative capacity of the bone marrow mononuclear cells in Duchenne muscular dystrophy patients in comparison with Non-DMD controls. This corroborates with the observation of Turk et al, who also depicted enhanced expression of specific muscle regenerative markers in DMD patients in comparison to Non-DMD cases [19].
There have been reports [20,21] stating autologus bone marrow transplantation as another mode of treating patients. Bone marrow being rich in stem cell population provided a good tool to enrich the regenerative capacity in DMD muscles. It has been shown that patients are given repeated shots of mononuclear cells isolated from bone marrow at regular intervals in order to maintain the strength of the muscles in DMD cases. In our study we have addressed this question and tried to explore the properties of the bone marrow from DMD and non- DMD cases along with their capacity in muscle regeneration in-vitro. This study would help in developing newer modalities enhancing the properties of the bone marrow stem cell, such that the patients need not be injected with mononuclear cells of bone marrow at regular intervals and would also give light in understanding the capacity of muscle stem cells or satellite cells, which would pave ways to understand the signalling mechanisms which could be exploited to enhance their regenerative capacity.
There are few possible target molecules which play the critical role in promoting the mono-nuclear cells isolated from bone marrow of DMD patients to differentiate in myogenic lineage specificity. Notch- Delta pathway is one of the plausible signalling pathway responsible for regulation of muscle regeneration in DMD cases. Previous work from Conboy et al [22,23] as well as Androutsellis- Theotokis A et al [24] and others, are strongly suggestive for a plausible role of Notch-Delta and Numb interplay. Understanding the interplay of the signalling pathways provides an insight in regulating the bone marrow cells as well as the satellite cells of the muscle tissue for enhancing the better regenerative potential. Bone Morphogenetic Protein (BMP) associated pathway proteins which are members of transforming growth factor-beta family, are turned on in –mdx mice, indicating a role in myogenic proliferation and differentiation [19]. Role of BMP has been well investigated in the neuromuscular junction in dystrophin deficient mice. Noordermeer’s lab has shown that BMP signaling is required at the neuromuscular junction in dystrophin deficient mice for releasing neurotransmitters. Expression analysis by Turk et al revealed BMP15 upregulation in dystrophin deficient mice. [19] Members of Epidermal Growth Factor-like (EGF-like) proteins are another family of proteins which remain un-elusive in muscle regeneration paradigm of mono-nuclear cells. Neuregulin 3 (Nrg3) and Erythroblastic leukemia viral oncogene homolog 4 (ErbB4) are couple of members belonging to EGF- like proteins which are up regulated in –mdx mice but not in control wild type mice.
Our study provides an important insight into the proliferative as well as differentiative capacity of the bone marrow mono-nuclear cells isolated from DMD and Non-DMD cases. The above findings answer the importance and advantages of autologus cell replacement therapy in patients with DMD. The basic understanding to the underlying mechanism would open up avenues for newer and better therapeutic modalities which would also eliminate side effects. Unraveling the signalling pathways playing role in regenerating the muscle may provide scope for enriching the muscle specific stem cell pool as well as enrich the satellite cell population in the muscle tissue.
Further studies to understand the difference in the mono-nuclear cells isolated from DMD and Non-DMD would provide interface for therapeutic intervention eliminating the need of repeated injection of isolated mono-nuclear cells. Ongoing research using stem cell as a cell replacement therapy in conjunction with exon skipping provides some clues for alleviating the DMD patient trauma. We hope our study would give scope on doing future research in not only alleviating the symptoms but also curing DMD.
References
- Kirschner J, Bönnemann CG. The congenital andlimb-girdle muscular dystrophies: sharpening the focus, blurring the boundaries. Archives of Neurology Journal.2004; 61:189-99.
- Emery AE. The muscular dystrophies. British Medical Journal 1998 Oct 10; 317(7164): 991-995.
- Bushby KMD et al. The multidisciplinary management of Duchenne muscular dystrophy.Current Paediatrics 2005; 15: 292-300.
- Louise V. B. Anderson, Katharine M. D. Bushby. Muscular Dystrophy: Methods and Protocols (Methods in Molecular Medicine).Totowa. New Jersey: Humana Press 2011; 111
- Haslett JN, Sanoudou D et al. Gene expression profiling of Duchenne muscular dystrophy skeletal muscle. Neurogenetics 2003; 4(4): 163-171
- Prior TW, Bridgeman SJ. Experience and strategy for the molecular testing of Duchenne muscular dystrophy.Journal of Molecular Diagnosis.2005 ;3: 317-326.
- Malmgren H, White I et al .PGD for dystrophingene deletions using Flourescence in situ hybridization. Molecular Human Reproduction 2006; 2(5): 353-356.
- Manzur AY, Kuntzer T, Pike M, et al. Glucocorticoid corticosteroids for Duchennemuscular dystrophy. Cochrane Database of Systematic Reviews 2004; 2: CD003725.
- Chakkalakal JV, Thompson J, Parks RJ, et al. Molecular, cellular, and pharmacological therapies for Duchenne/Becker muscular dystrophies. The Federation of American Societies for Experimental Biology Journal 2005; 19(8): 880-891.
- Steve W, Susan F. Exon skipping and Duchennemuscular dystrophy: Hope, hype and how feasible? Neurology India 2008; 56(3): 225-394.
- Dellavalle M, Sampaolesi R, Tonlorenziet al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells, Nature Cell Bioliogy 2007; 9: 255-267.
- Schenk S, Mal N, Finan A et al. Monocyte chemotactic protein-3 is a myocardial mesenchymalstem cell homing factor. Stem Cells 2007; 25: 245- 251.
- Seeger FH, ZeiherAM, Dimmeler S. Cellenhancement strategies for the treatment of ischemic heart disease. Nature Clinical Practice Cardiovascular Medicine 2007; 4: S110-S113
- Shyu WC, Lee YJ, Liu DD, Lin SZ, Li H. Hominggenes. Cell therapy and stroke.Frontiers in Bioscience.2006; 11: 899-907.
- Hogaboam CM, Carpenter KJ, Schuh JM, ProudfootAA, Bridger G, Buckland KF. The therapeutic potential in targeting CCR5 and CXCR4 receptors in infectious and allergic pulmonary disease. Pharmacology and Therapeutics Journal 2005; 107: 314-328.
- Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell 2008; 132: 612-630.
- Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007; 25: 2739-2749.
- Wolf D, Wolf AM. Mesenchymal stem cells as cellular immunosuppressants. Lancet 2008; 371: 1553-1554.
- Turk R, Sterrenburg E, de Meijer EJ, van OmmenG-JB, den Dunnen JT, Hoen PAC. Muscleregeneration in dystrophin-deficient mdx mice studied by gene expression profiling. BMC Genomics 2005; 6: 98-112.
- Palermo AT, LaBarge MA, DoyonnasR, Pomerantz J, Blau HM, Bone marrow contribution to skeletal muscle: a physiological response to stress. Developmental Biology 2005; 279: 336- 344.
- Risbud MV, Shapiro IM, Guttapalli A, Di Martino A, Danielson KG, Beiner JM et al. Osteogenicpotential of adult human stem cells of the lumbar vertebral body and the iliac crest. Spine 2006; 31: 83-89.
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Developmental Cell 2002; 3: 397-409.
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL,Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature.2005; 433:760-764.
- Androutsellis-Theotokis A. et al. Notch signaling regulates stem cell numbers in vitro and in vivo. Nature 2006; 442: 823-826.