ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2012) Volume 23, Issue 1
Fibrinogen-like protein 2 Gene silencing activates angiopoietin/Tie system and induces Myocardial Microvascular Endothelial Cells proliferation and cell migration
Zhenzhong Zheng1,2, Liang Wang1,Youpin Wu1, Huifang Wang1, Dengpeng Gao1, Yuya Fa1, Yunfeng Wei1, Menghong Wang1, Zeqi Zheng1,Jintian Peng1
117# Yongwaizheng street,Department of cardiology,the first afficiated Hospital of Nanchang University, Nanchang,Jiangxi,330006,China
- Corresponding Author:
- Zhenzhong Zheng
117# Yongwaizheng Street
Department of cardiology
The first Affiliated Hospital of Nanchang
University, Nanchang, Jiangxi,330006,China
Email: zhengzhenzhong68@yahoo.cn
Accepted date: October 15 2011
Fibrinogen-like protein 2 (Fgl2)/fibroleukin has recently been identified as a new member of fibrinogen-related protein superfamily, with the serine protease activity. Fgl2 functions as a novel immune coagulant with the ability to generate thrombin directly,and may be involved in angiogenesis. Cells proliferation and cell migration are critical steps in angiogenesis. In the present study, we investigated the expression of hFgl2 mRNA after Fgl2 RNAi transfection in myocardical microvascular endothelial cell,we found that Fgl2 mRNA expression decreased significantly.Results also showed that the proliferation and migration of myocardical microvascular endothelial cell increased significantly after Fgl2 RNAi transfection. The decreased Fgl2 mRNA levels,result in increased Tie2, Ang1, and Ang2 gene expression in myocardical microvascular endothelial cell.This study indicated that Fgl2 Gene silencing activates angiopoietin/Tie system and induces Myocardial Microvascular Endothelial Cells proliferation and cell migration.
Keywords
Fibrinogen-like protein 2, angiopoietin/Tie system, cell proliferation, cell migration
Introduction
Fibrinogen-like protein 2 (Fgl2)/fibroleukin has recently been identified as a new member of fibrinogen-related protein superfamily, with the serine protease activity. The biological activity of Fgl2 prothrombinase, similar to coagulating factor Xa, can directly catalyze prothrombinase into activated thrombinase, thereby, initiating a cascade coagulating reaction[1]. Several studies indicate that Fgl2 is involved in pathological changes such as immune coagulation, fibrin deposition, and micro-thrombus[2]. In addition to its primary role in homeostasis and blood coagulation, thrombin is a potent mitogen that dramatically increases the growth and metastasis potential of tumor cells. Fgl2 functions as a novel immune coagulant with the ability to generate thrombin directly,and may be involved in angiogenesis[3].
We have verified that angiogenesis may be beneficial in the treatment of ischemia heart diseases[4].As we known,target thrombus and angiogenesis are very effective strategy to treat ischemia heart diseases,so Fgl2 is a potential target on both angiogenesis and thrombus.
Cell proliferation and migration are the major events of angiogenesis[5,6],and angiopoietin/Tie system plays important role in cell proliferation and migration. Therefore, we propose that Fgl2 may be involved in cell proliferation and migration. Up to now,we have not any information about it,we did not know the effect of Fgl2 on proliferation and migration of Myocardial Microvascular Endothelial Cells (MMVECs) and the mechanism involved.
In the present study, we constructed a lentiviral RNA interferenced vector of human fibrinogen-like protein 2(hFgl2) gene. Myocardial Microvascular Endothelial Cells (MMVECs) were transfected by lentiviral vector.To investigate how Fgl2 gene silence affects the proliferation and migration of MMVECs and whether angiopoietin/Tie system activation involved or not.
Materials and methods
This study is an experimental study and has been approved by the the first afficiated Hospital of Nanchang University Research and Ethical Committee. The study duration is from 12 september 2009 to 30 May 2011, approximately 20 months.
Materials
Culture medium, fetal calf serum (FCS), glutamine and antibiotics were from Hyclone Company (USA); EC growth supplement, gelatin, trypsin, amphotericin B and hydrocortisone were from Sigma (Germany); RNAtocDNA Kit, Avidin Biotin Complex were purchased from Nanjing JianChen bioengineering institute. Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Gibco, USA. Trizol reagent was purchased from Invitrogen Ltd (USA); Mouse murine leukemia virus reverse transcriptase was purchased from Promega (USA). pGCSIL-Fgl2 pGC-LVp Helper 1.0 pHelper are provided by Jikai gene company(China). FVIII RA antibody was purchased from Solarbio company(China)
Myocardical Microvascular Endothelial Cell Cultures
Hearts were excised from Sprague-Dawley (SD) rats of seven days old under aseptic conditions. Cardiac ventricles were cleaned by sterilized Hank’s solution and then cut into pieces. A homogenate of ventricles was suspended and filtered by sequential 200 μm and 60 μm microfiltration. The filtered cells were cultured in culture flasks (75 cm2) at 37 °C in a humidified atmosphere with 5% CO2 in endothelial cell growth medium (ECGM), as supplied by the manufacturer, supplemented with 10 ng/mL human recombinant epidermal growth factor, 1 μg/mL hydrocortisone, 50 μg/mL gentamicin, 50 μg/mL amphotericin B, 12 μg bovine brain extract and 2% v/v fetal calf serum (FCS) for three days and used in passages 3–6.
RNAi Design and Preparation and transfection of siRNAs
Fgl2 RNAi or GFP virus were provided by Jikai Company. Briefly,the nucleotide sequences of hFgl2(NM_006682) were found in the GeneBank.Tatget sequence of Fgl2 gene (Forward:5′- CCGGCACGTCCAGTTCAACATCTAATTCAAGAGATT AGATGTTGAACTGGACGTGTTTTTG- 3′,Reverse:5′- AATTCAAAAACACGTCCAGTTCAACATCTAATCTCTTGAATTAGATGTTGAACTGGACGTG- 3′.) could be effectively scilenced with RNA interference were confirmed. The cDNA containing both sense and antisense Oligo DNA fragements of target sequences were designed and cloned into the pGCSIL-GFP vector which was digested by AgeI/EcoRI.The obtained lentiviral vector containing Fgl2shRNA was confirmed by digestion and sequencing. Using lentiviral vector pHelper 1.0 pHelper2.0 pGC-LVFgl2 to transfect 293T cells,then the lentivirus were collected.The titer of virus was tested according to the expression level of GFP. Titers of the virus stocks were routinely 1×109 transduction units (TU) ml−1.
Real-time RT-PCR
Real-time reverse transcription-polymerase chain reaction (PCR) was used to quantify mRNA expression.Fgl2 RNAi or GFP virus infected Myocardical Microvascular Endothelial Cell were harvest at 96 hr after infection. Total RNA was extracted from the infected cell samples using Trizol reagent Kit according to the manufacturer's protocol. Viral mRNA copies were measured by SYBR green M×3000 Real-Time PCR System (Stratagene,USA),using primers Fgl2-Forward:5′- AGTCGCTCCAACTGGTA AAT-3′,Fgl2-Reverse:5′- TCTGTAGGTCCCACTGCTTC-3′;Ang-1-Forward: 5′- TTT TGTGCTGGGTCTGGT-3′,Ang-1-Reverse:5′- CTTGATGCTGCCCTTGTT-3′;Ang-2-Forward:5′- GCGACCCCTTCAACTCTTG-3′,Ang-2-Reverse:5′- GTATCTGGCATCCCGACC-3′;Tie-2-Forward:5′- GCGACCCCTTCAACTCTTG-3′,Tie-2-Reverse:5′- TGACCCAGATGTTTGAGACCTT-3′;β-actin- Forward:5′-TGACCCAGATGTTTGAGACCTT-3′, β- actin-Reverse: 5′-CGGAGTCCATCACAATGCCAGT- 3′. The reactions were performed at 95°C 10 mins, 40 cycles of 95°C 1 min, 60°C 1 min, 72°C 1 min, followed by melting curve analysis according to instrument documentation. All reactions were done in triplicates and the results were normalized by β-action.
Proliferation assay
To investigate the effects of Fgl2 RNAi on myocardical microvascular endothelial cell proliferation, myocardical microvascular endothelial cell were infected with Fgl2 RNAi,, or GFP virus in 6-well plates in triplicate, and 5 days later the cells were trypsinized and seeded in 96-well plates in triplicate(1×104 cells/well). After attachment, the cells were exposed to DMEM with 0.5% FBS for 48 h and then the effects of Fgl2 RNAi on myocardical microvascular endothelial cell proliferation were evaluated using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. Briefly, medium was removed and replaced with medium containing 5 mg/ml MTT and incubated for 4 h. The mediumwas then aspirated, and the product was solubilized with dimethyl sulfoxide (DMSO). Absorbance was measured at 570 nm for each well using a microplate reader according to the manufacturer’s protocol.
Migration assay
Cell migration assay was performed using boyden transwell chambers.Briefly, 200 μl DMEM containing 10% FBS was added to the bottom well. Cells were resuspended in the appropriate buffer at a concentration of 5×105 cells/ ml and 500 μl cell suspension was added to the top well of transwell chambers.Between the bottom well and the top well, there was the filter (pore size: 8 μm). After incubation at 37 ◦C in 5% CO2 condition, the cells that had not migrated were removed from the upper surface of the filters using cotton swabs, and those that migrated to the lower surface of the filters were fixed in methanol and stained with 0.1% crystal violet. Migration was determined by counting the cell number with a microscope at 400×. Five visual fields were chosen randomly for each assay. The average number of the migrating cells in five fields was taken as the cell migration number of the group. The assays were repeated three times. Data are presented as relative migration (number of cells/high power field) and represent mean±standard error.
Statistics
Values presented are the mean±S.E.M. Comparisons between two groups were performed using Student’s t-test; three or more groups were compared by analysis of variance followed by the Newman–Keuls test. P values less than 0.05 were considered significant.
Results
Fgl2 RNAi decreases the expression of Fgl2 mRNA
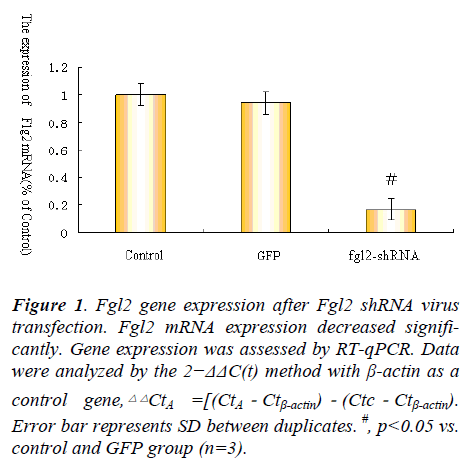
To observe the expression of Fgl2 mRNA after myocardical microvascular endothelial cell infected with Fgl2 RNAi or GFP virus,qPCR was used to determine the expression of Fgl2 mRNA expression.The results showed that myocardical microvascular endothelial cell infected with Fgl2 RNAi resulted in decreased expression of Fgl2 mRNA compared to control and group infected with GFP virus (as a control for transfection efficiency) (Fig. 1).
Figure 1. Fgl2 gene expression after Fgl2 shRNA virus
transfection. Fgl2 mRNA expression decreased significantly.
Gene expression was assessed by RT-qPCR. Data
were analyzed by the 2−ΔΔC(t) method with β-actin as a
control gene,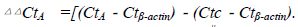 .Error bar represents SD between duplicates. #, p<0.05 vs.
control and GFP group (n=3).
.Error bar represents SD between duplicates. #, p<0.05 vs.
control and GFP group (n=3).
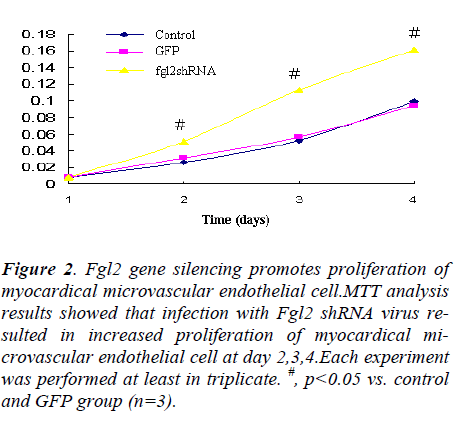
Figure 2. Fgl2 gene silencing promotes proliferation of myocardical microvascular endothelial cell.MTT analysis results showed that infection with Fgl2 shRNA virus resulted in increased proliferation of myocardical microvascular endothelial cell at day 2,3,4.Each experiment was performed at least in triplicate. #, p<0.05 vs. control and GFP group (n=3).
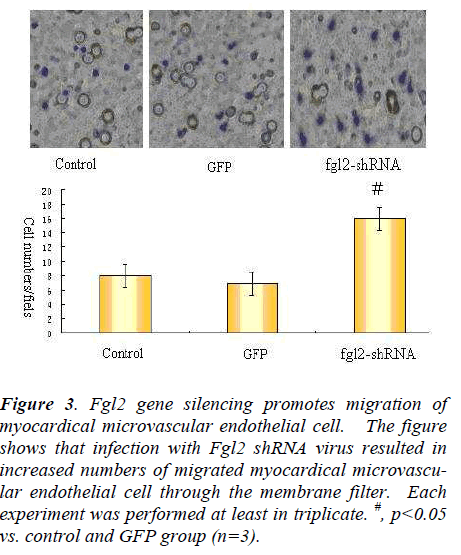
Figure 3. Fgl2 gene silencing promotes migration of myocardical microvascular endothelial cell. The figure shows that infection with Fgl2 shRNA virus resulted in increased numbers of migrated myocardical microvascular endothelial cell through the membrane filter. Each experiment was performed at least in triplicate. #, p<0.05 vs. control and GFP group (n=3).
MTT analysis
In order to determine the proliferative effects using MTT assay. Myocardical microvascular endothelial cell infected with Fgl2 RNAi resulted in decreased expression of Fgl2 mRNA and significantly increase myocardical microvascular endothelial cell proliferation compared to the control and group infected with GFP virus (Fig. 2).The effects were significant at 2,3,4 days,and the effect reach the maximum at day 4, which suggested that Fgl2 RNAi can promotes cell proliferation.
Migration analysis
We determined the migration effects of Fgl2 RNAi transfection using boyden chamber assay. In transwell boyden chamber assay,Infection with Fgl2 RNAi, stimulated myocardical microvascular endothelial cell migration (Fig. 3). These data suggest that Fgl2 play important roles in regulating migration of myocardical microvascular endothelial cell.
qPCR analysis of Ang1 Ang2 Tie-2
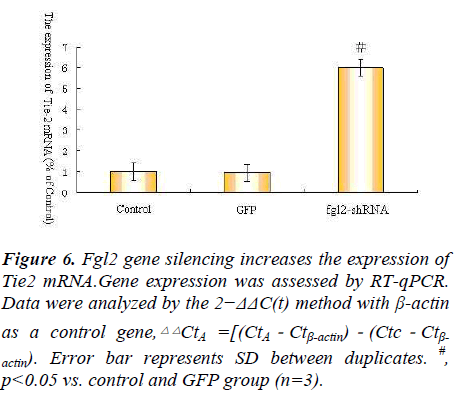
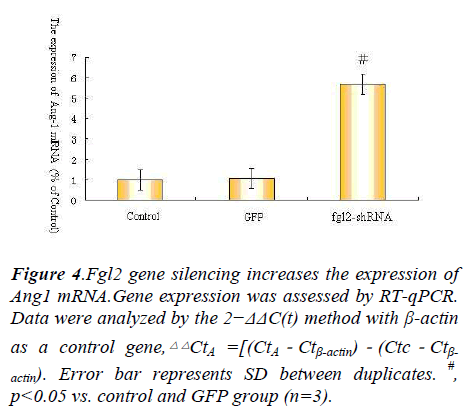
As we all known Ang1 Ang2 Tie-2 play important roles in angiopoietin/Tie system ,so we explore angiopoietin/ Tie system activation in the process, so we observe the expression of Ang1 Ang2 Tie-2 mRNA after Fgl2 RNAi transfection.The results showed that Fgl2 RNAi increase the expression of Ang1 Ang2 Tie-2 mRNA(Fig. 4.5.6).
Disscusion
In the present study, we investigated the expression of hFgl2 mRNA after Fgl2 RNAi transfection in myocardical microvascular endothelial cell,we found that Fgl2 mRNA expression decreased significantly after Fgl2 RNAi transfection. In addition,the proliferation and migration of myocardical microvascular endothelial cell increased significantly at the same time.The results showed that Fgl2 is a target to regulate cell proliferation and migration, which indicated that Fgl2 is involved in angiogenesis.
Endothelial cell proliferation and migration play critical roles in angiogenesis [7]. Angiogenesis is the formation of new blood vessels from pre-existing vessels, a physiological or pathological neo-vascularization process in response to tissue ischemia and tumor growth or metastasis, which is complex and involves several discrete steps such as extracellular matrix degradation, proliferation and migration of endothelial cells, and morphological differentiation of endothelial cells to form tubes. Many growth factors and hormones have been shown to regulate cell proliferation, migration and angiogenesis, including Ang1,Ang2,Tie-2,and etc[8,9].
As we known the receptor tyrosine kinase Tie2 is essential for vascular development and maintenance and interacts with a family of ligands known as the angiopoietins. Signaling through Tie2 is tightly controlled. The angiopoietin family contains members with full agonist activity, the best characterized of which is Ang1, as well as, unusually, members with low or partial activity, notably Ang2 [10,11]. This partial agonist activity of Ang2 allows the ligand to act as an apparent antagonist to Ang1, by competing with Ang1 for Tie2 binding and thereby re placing the full agonist activity of Ang1 with the much lower activity of Ang2 [10,11].
The angiopoietin/Tie system plays critical roles in embryonic vascular development and maintenance of normal adult vasculature. [12,13,14] Previous study demonstrate that over expression of Ang1 in mice can increase vascularization in the skin, and activation of angiopoietin/Tie2 signaling pathway can lead to excessive angiogenesis. [15,16.]. Defects in Tie2 are known to affect vascular development in humans. An activating mutation in the intracellular kinase domain of human Tie2 gene has been implicated in one form of inherited cutaneo-mucosal venous malformations, demonstrating the critical function of Tie2 in proper vascular development. [17] Whether the angiopoietin/ Tie system is involved in the process which Fgl2 control cell proliferation and migration is still unknown. This study has identified the Fgl2 as molecular regulators associated with the pathogenesis of cell migration and proliferation,which is involved in angiogenesis. The decreased Fgl2 mRNA levels result in increased Tie2, Ang1, and Ang2 gene expression in myocardical microvascular endothelial cell may contribute to the abnormal growth and remodeling of vasculatures in ischemia heart diseases.The possible mechanism may be that the angiopoietin/Tie system activation is involved in the process.
In summary, Targeting Fgl2 gene may prove to be effective anti-angiogenic or angiogenic therapy for preventing ischemia heart diseases. The preliminary data need to be well discussed and further study surely continued. Further study of Fgl2 will provide clues for better understanding its function and may help uncover novel therapeutic strategies for treating ischemia heart diseases.
Acknowledgements
This work was supported by a grant from the National Nature Science Foundation of China (No. 30960119) and the Technology Support project of Jiangxi province (NO. 2010BSA12000).
References
- Levy GA, Liu M, Ding J, Yuwaraj S, Leibowitz J, Marsden PA, Ning Q, Kovalinka A, Phillips MJ. Molecular and functional analysis of the human prothrombinase gene (HFGL2) and its role in viral hepatitis. Am J Pathol. 2000;156:1217–1225.
- Rickles FR, Shoji M, Abe K. The role of the hemostatic system in tumor growth, metastasis, and angiogenesis: tissue factor is a bifunctional molecule capable of inducing both fibrin deposition and angiogenesis in cancer. Int J Hematol. 2001;73:145–150.
- Su K, Chen F,Yan WM,Zeng QL,Xu L,Xi D,Pi B,Luo XP,Ning Q.Fibrinogen-like protein 2/fibroleukin prothrombinase contributes to tumor hypercoagulability via IL-2 and IFN-gamma. World J Gastroenterol. 2008;14(39):5980-5989.
- Zheng Z, Liu Z.CD151 gene delivery activates PI3K/Akt pathway and promotes neovascularization after myocardial infarction in rats.Mol Med. 2006;12(9- 10): 214-20.
- Zheng ZZ, Liu ZX.CD151 gene delivery increases eNOS activity and induces ECV304 migration, proliferation and tube formation.Acta Pharmacol Sin. 2007 ;28(1): 66-72.
- Zheng ZZ, Liu ZX.Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates CD151-induced endothelial cell proliferation and cell migration.Int J Biochem Cell Biol. 2007;39(2):340-8.
- Chen JX, Lawrence ML, Cunningham G, Christman BW, Meyrick B. HSP90and Akt modulate Ang-1- induced angiogenesis via NO in coronary artery endothelium. J. Appl. Physiol. 2004, 96(2), 612–620.
- Zhang J,Fukuhara S,Sako K,Takenouchi T,Kitani H,Kume T,Koh GY,Mochizuki N.Angiopoietin-1/Tie2 signal augments basal Notch signal controlling vascular quiescence by inducing delta-like 4 expression through AKT-mediated activation of beta-catenin.J Biol Chem.2011 11;286(10): 8055-8066.
- Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, De Palma M.Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells.Cancer Cell. 2011;19(4):512-26.
- Bogdanovic E., Nguyen V.P.K.H., Dumont D.J. Activation of Tie2 by angiopoietin-1 and angiopoietin- 2 results in their release and receptor internalization.J. Cell Sci. 2006;119:3551-3560.
- Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium.Mol. Cell Biol. 2009;29:2011-2022.
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y: Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 1995, 376:70-74.
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996, 87:1171-1180.
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD.Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277:55-60.
- Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD.Increased vascularization in overexpressing angiopoietin-1. Science 1998, 282:468-471.
- Lin P, Polverini P, Dewhirst M, Shan S, Rao PS, Peters K. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J Clin Invest 1997, 100:2072-2078.
- Vikkula M, Boon LM, Carraway KL 3rd, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell 1996, 87:1181-1190.
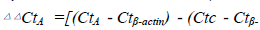 actin). Error bar represents SD between duplicates. #,
p<0.05 vs. control and GFP group (n=3).
actin). Error bar represents SD between duplicates. #,
p<0.05 vs. control and GFP group (n=3).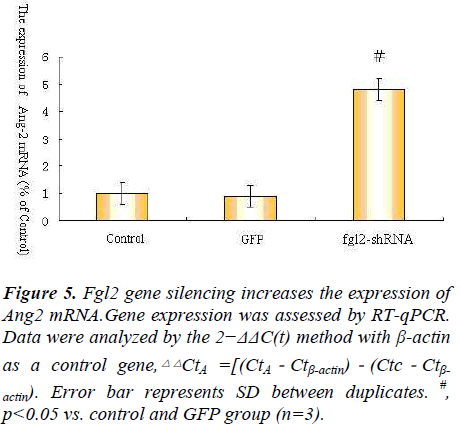
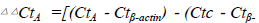 actin). Error bar represents SD between duplicates. #,
p<0.05 vs. control and GFP group (n=3).
actin). Error bar represents SD between duplicates. #,
p<0.05 vs. control and GFP group (n=3).