ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2020) Volume 31, Issue 1
GC-MS analysis and pharmacological potentials of Neurada procumbens.
Department of Pharmaceutical Chemistry, Bahauddin Zakariya University, Multan, 60800, Pakistan
- *Corresponding Author:
- Muhammad Munawar Hayat
Department of Pharmaceutical
Chemistry Bahauddin Zakariya University
Pakistan
Accepted Date: December 27, 2019
Muhammad Munawar Hayat*, Muhammad Uzair
Department of Pharmaceutical Chemistry, Bahauddin Zakariya University, Multan, 60800, Pakistan
Introduction
The Neurada procumbens is a desert plant belongs to family Neuradaceae and traditionally used a nerve tonic and cooling agent with the name of herbal preparation “Thadal” in summer by local people. Its folkloric uses are as anthelmintic, anticonvulsant, laxative, infestinal problems, eczema, infections, rheumatism and sexual problems [1]. The leaves and fruits of the plant are used for medicinal purposes and whole plant is a source of fodder for camel [2]. It is an annual prostrate densely tomentose herb. The local name of Neurada procumbens is “Chapperi Booti” and “Als’dan”. According to Flora of West Pakistan, there is only single species distributed in Pakistan from the genus Neurada [3]. The influence of aqueous extract of N. procumbens when administered orally increases the blood pressure of rats. The study provides that elevation of blood pressure was due to vasoconstriction on the aortic strips of rats in vitro [4]. It is also reported that ethanolic extract of N. procumbens reduced the blood pressure in rabbits [5]. Quinone oxidoreductase 1 inducer activity of plant is also reported [6]. Recently, some flavonoids are isolated from N. procumbens in Egypt [7]. Literature survey revealed that there is too much diverse folkloric use of the plant in residants of Cholistan desert but little work regarding biological screening and enzymatic inhibition studies of N. procumbens has been done. Therefore, it was need to explore the therapeutical potential of the plant. The objectives of present study were to investigate the secondary metabolites, chemical constituents and enzymatic or biological activities. Despite the multiple ethanomedicinal uses of N. procumbens in diarrhea, dysentery, diabetes, inflammation, ulcer, edema, asthma, oxidative stress and skin problems, no research work is available with respect to its effectiveness in these diseases. The present work on the two crude extracts of the plant N. procumbens was undertaken for various biological and enzyme inhibition activities to scientifically rationalize the traditional uses and also includes the phytochemical screening or evaluation of the plant.
Materials and Methods
Collection and extraction of plant Objective: Neurada procumbens L is a medicinal herb with diverse folk history and is traditionally used to treat diarrhea, dysentery, diabetes, eczema, anticonvulsant, rheumatism, laxative, infestinal and gastrointestinal problems, sexual problems, anthelmintic, infections and respiration function. The aim of this study was to prove the uses of plant N. procumbens in gastrointestinal and respiratory disorders scientifically.
Method: The phytoconstituents were identified by GC-MS technique. In this study, twelve (12) different kinds of in vitro biological and enzyme inhibition activities were performed to explore the diverse folkloric use of the plant scientifically.
Results: On phytochemical screening, it was proved that plant N. procumbens is rich source of flavonoids as dichloromethane and methanol extracts have 143.45 ± 1.25 and 200.69 ± 1.97 mg QE/g of extract, respectively. By GC-MS analysis, nine (9) compounds in dichloromethane extract and six (06) in methanol extract were detected. The dicloromethane extract of whole plant of N. procumbens showed anti-lipooxygenase, anti-chymotripsin, anti- alpha glucosidase, anti-urease and anti-tyrosinase activities, whereas methanol extract showed antioxidant, anti-alpha glucosidase, anti-urease, anti-tyrosinase, anticarbonic anhydrase II, spasmolytic and bronchorelaxant activities.
Conclusion: The study proofs the folk medicinal use of N. procumbens to treat diabetes, diarrhea, and improvement in gastrointestinal and respiration functions which justifies the pharmacological importance of plant. Moreover, this study can be considering a solid back ground for further in vivo studies.
Keywords
Alpha glucosidase, Urease, Glass chromatography-mass spectrometry, Neurada procumbens
Introduction
The Neurada procumbens is a desert plant belongs to family Neuradaceae and traditionally used a nerve tonic and cooling agent with the name of herbal preparation “Thadal” in summer by local people. Its folkloric uses are as anthelmintic, anticonvulsant, laxative, infestinal problems, eczema, infections, rheumatism and sexual problems [1]. The leaves and fruits of the plant are used for medicinal purposes and whole plant is a source of fodder for camel [2]. It is an annual prostrate densely tomentose herb. The local name of Neurada procumbens is “Chapperi Booti” and “Als’dan”. According to Flora of West Pakistan, there is only single species distributed in Pakistan from the genus Neurada [3]. The influence of aqueous extract of N. procumbens when administered orally increases the blood pressure of rats. The study provides that elevation of blood pressure was due to vasoconstriction on the aortic strips of rats in vitro [4]. It is also reported that ethanolic extract of N. procumbens reduced the blood pressure in rabbits [5]. Quinone oxidoreductase 1 inducer activity of plant is also reported [6]. Recently, some flavonoids are isolated from N. procumbens in Egypt [7].
Literature survey revealed that there is too much diverse folkloric use of the plant in residants of Cholistan desert but little work regarding biological screening and enzymatic inhibition studies of N. procumbens has been done. Therefore, it was need to explore the therapeutical potential of the plant. The objectives of present study were to investigate the secondary metabolites, chemical constituents and enzymatic or biological activities. Despite the multiple ethanomedicinal uses of N. procumbens in diarrhea, dysentery, diabetes, inflammation, ulcer, edema, asthma, oxidative stress and skin problems, no research work is available with respect to its effectiveness in these diseases. The present work on the two crude extracts of the plant N. procumbens was undertaken for various biological and enzyme inhibition activities to scientifically rationalize the traditional uses and also includes the phytochemical screening or evaluation of the plant.
Materials and Methods
Collection and extraction of plant
The whole plant of N. procumbens was collected freshly from Cholistan desert of Bahawalpur Punjab, Pakistan in April 2013 and identified by research officer of Cholistan Institute of Desert Studies, The Islamia University of Bahawalpur, Pakistan. The plant herbarium sheet was deposited for future reference and voucher specimen number 3477/CIDS/IUB was allocated.
The whole plant was dried under shade for 15 days and ground in fine powder by crushing mill. The plant material powder approximately 850 g was macerated in dichloromethane for 24 hours, the process was repeated for three days and then same in methanol for three days. The both dichloromethane and methanol extracts are concentrated separately in solid residue by using the buchi rotary evaporator under reduce pressure. The dichloromethane extract (15.6 g) and methanol extract (14.2 g) were prepared and there yields were 1.8% and 1.6%, respectively.
Chemicals
Lipooxygenase (EC 1.13.11.12), urease (EC 3.5.1.5), alpha glucosidase (EC 3.2.1.20), chymotrypsin (EC 3.4.21.1), tyrosinase (EC 1.14.18.1), DDPH, Thiourea, urea, acarbose, kojic acid, N-succinyl phenyl-alanine-P- nitroanilide, baicalein, chymostatin, eserine, quercetin and gallic acid were purchased from Sigma-Aldrich Co. St. Louis, Mo. USA, Merck and Fluka companies. The dichloromethane and methanol were of highest purity. Na2CO3, NaNO, Sodium hydroxide, hydrochloric acid, Na2PO4, KH2PO4, Tris-HCl buffer, DTNB [5,5-dithiobis (2-nitrobenzoic acid)], DMSO, Folin Ciocalteu reagent, phenol hypochlorite reagent and alkali reagent were of biochemical/analytical grade.
Instrumentation
The instruments synergy HT BioTek® USA 96 microplate reader, Buchi rotary evaporator with vacuum pump, Spectrophotometer (Shimadzo, Japan), Gas chromatography-mass spectrometry (GC Agilent system, USA), EZ-Fit Enzyme Kinetics software (Perrella Scientific Inc. Amherst, USA) and Powerlab Data Acquistion System (AD instrument, Sydney, Australia) were used in this study.
Phytochemical screening
Detection of secondary metabolites: The dry powder of whole plant N. procumbens was investigated for the presence of secondary metabolites in plant material of whole plant [8]. The flavonoids, glycosides, cardiac glycosides, sterol and terpenes were present.
Total phenol contents determination: Total phenol contents in the both extracts of N. procumbens were calculated by folin-ciocalteu reagent (FCR) by using the reported literature method [9]. The 20 μl of plant extract solution (0.5 mg/mL) was added with folin-ciocalteu reagent (90 μl, 1:10 ratio made with water) in micro plate reader. After that added aqueous sodium carbonate (90 μl, w/v) in it. Total phenol contents were determined at wave length 725 nm. The same above mentioned process was repeated for galic acid (GA) standard solution and the calibration curve was developed. On the basis of measured absorbance, the concentration of total phenol contents was quantified (mg/ml) by using calibration curve. The total phenol contents in both extracts were expressed in term of gallic acid equivalent (mg of GA per gram of extract) in Table 1.
| S. No. | Extract code | Total Phenol contents (mg GA/g of extract) |
Total Flavonoid contents (mg QE/g of extract) |
|---|---|---|---|
| 1 | NPD | ------- | 143.45 ± 1.25 |
| 2 | NPM | 12.37 ± 0.12 | 200.69 ± 1.97 |
Table 1. Total phenol and flavonoid contents results in dichloromethane and methanol extracts of N. procumbens.
Total flavonoid contents determination: Total flavonoid contents in both extracts of N. procumbens were analyzed by the reported method [10]. The plant extract solution (20 μl) was mixed with 80 μl de-ionized water in in microplate. Sodium nitrous oxide (5%) solution (6 μl) was added followed by the addition of aluminum chloride (10%) solution (16 μl) and sodium hydroxide (4%) solution (68 μl). After 20 minutes incubation, the absorbance value was read at 510 nm. Above mentioned procedure was repeated for quercetin (QE) standard solution for construction of calibration curve. Total flavonoid contents were calculated (mg/ml) by using calibration curve. The contents of the flavonoids in both extracts were expressed in term of quercetin equivalent (mg QE per gram of extract) in Table 1.
Gas chromatography-mass spectrometry (GC-MS)
GC-MS analysis is used for identification of chemical constituents present in the both extracts of plant N. procumbens. It has great importance in drug detection and identification of unknown phytochemical constituents present in the medicinal plants. So, GC-MS analysis included in the study to investigate the chemical constituents of the selected medicinal plant N. procumbens. GC-MS analysis [11,12] was performed on GC Agilent system (B 7890) with mass spectrometer detector (MSD-5977A) employing the following condition: Column HP-5 MS, size 30 m × 0.25 mm, 0.25 μ, composed of 100% dimethyl poly siloxane. The source temperature for ionization was set at 250°C. The 2 μl of dichloromethane and methanol extracts of whole plant of N. procumbens were used in GC-MS analysis. The GC-MS results revealed the presence of fifteen (15) compounds in two different extracts of N. procumbens. The name, molecular formula, molecular weight and structure of the compounds are given in Tables 2 and 3.
| S. No. | Phytoconstituent | Mol. Formula | Mol. Weight | Structure |
|---|---|---|---|---|
| 1 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | C20H40O | 296 |  |
| 2 | 12-methyl-E,E-2,13-octadecadien-1-ol | C19 H36O | 280 | 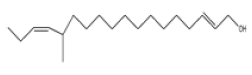 |
| 3 | 13-Heptadecyn-1-ol | C17H32O | 252 |  |
| 4 | n-Hexadecanoic acid | C16H32O2 | 256 | 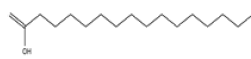 |
| 5 | 9,12,15-octadecatrie- noic acid, 2,3-dihydroxy propyl ester, (Z,Z,Z)- | C21H36O4 | 352 | 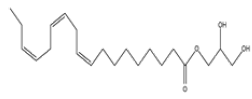 |
| 6 | Phytol | C20H40O | 296 | 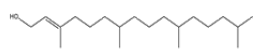 |
| 7 | Trans-13-Octadecenoic acid | C18H34O2 | 282 | 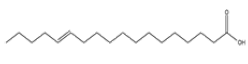 |
| 8 | Oleic acid | C18H34O2 | 282 |  |
| 9 | Ethyl iso-allocholate | C26H44O5 | 436 | 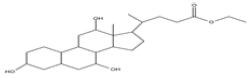 |
Table 2. Phytochemical constituents identified by GC-MS in dichloromethane extract of N. procumbens.
| S. No. | Phytoconstituent | Mol. Formula | Mol. Weight | Structure |
|---|---|---|---|---|
| 1 | Pterin-6-carboxylic acid | C7H5N5O3 | 207 | 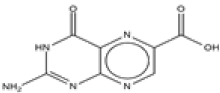 |
| 2 | Ergosta-5,22-dien-3-ol,acetate, (3β,22E)- | C30H48O2 | 440 | 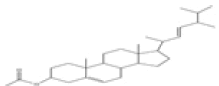 |
| 3 | 5H- cyclopropa [3, 4] benz [1,2–e] azulen–5- one, 9, 9a-bis (acetyloxy)-1, 1a, 1b, 2, 4a, 7a, 7b, 8, 9, 9a-decahydro-2, 4a, 7b-trihydroxy-3-(hydroxyl methyl)-1,1,6,8-tetra methyl-, [1aR -(1aα,1bβ, 2β,4aβ,7aα,7bα,8α,9β,9aα]- | C24H32O9 | 464 | 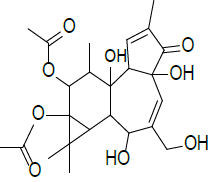 |
| 4 | Hexadecanoic acid, methyl ester | C17H34O2 | 270 |  |
| 5 | 7,10-octadecadienoic acid | C19 H34O2 | 294 |  |
| 6 | 10-octadecenoic acid, methyl ester | C19 H36O2 | 296 | 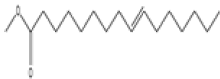 |
Table 3. Phytochemical constituents identified by GC-MS in methanol extract of N. procumbens.
Biological and enzyme inhibition activities
Antioxidant activity: The DPPH assay is very simple, was performed by reported method [13]. The test solution (10 μl) followed by the addition of 100 μM methanol DPPH solution (90 μl) to make volume of 100 μl in microplate. The mixture was incubated for about half an hour. The absorbance was reduced which was measured at 517 nm using the equipment plate reader. The standard compound is quercetin in this assay. The results are given in Table 4.
| S. No | Biological and enzyme inhibition activities | Standard / control | DCM extract | Methanol extract | |||
|---|---|---|---|---|---|---|---|
| Inhibition (%) | IC50 ( µMoles) | Inhibition (%) | IC50 ( µMoles) | Inhibition (%) | IC50 ( µMoles) | ||
| 1 | Antioxidant activity | 83.68 ± 3.76 Quercetin | 16.96 ± 0.14 Quercetin | 1.28 ± 1.66 | Inactive | 75.38 ± 3.16 | 88.30 ± 1.21 |
| 2 | Acetylcholin-esterase inhibition activity | 91.27 ± 0.17 Eserine | 0.04 ± 0.001 Eserine | -38.27 ± 0.48 | Inactive | 23.88 ± 0.01 | Inactive |
| 3 | Butyrylcholi-nesterase inhibition activity | 91.27 ± 0.17Eserine | 0.04 ± 0.001 Eserine | -38.27 ± 0.48 | Inactive | 23.88 ± 0.01 | Inactive |
| 4 | Lipoxygenase inhibition activity | 93.79 ± 1.27 Baicalein | 22.47 ± 0.04 Baicalein | 44.60 ± 0.23 | 524.60 ± 1.02 | 31.70 ± 0.76 | Inactive |
| 5 | Chymotripsin inhibition activity | 93.50 ± 0.91 Chymostatin | 8.24 ± 0.11 Chymostatin | 84.38 ± 0.05 | 115.71 ± 0.03 | 18.60 ± 0.03 | Inactive |
| 6 | Alpha glucosidase inhibition activity | 92.23 ± 0.14 Acarbose | 38.25 ± 0.12 Acarbose | 95.49 ± 0.65 | 24.32 ± 0.62 | 99.45 ± 0.58 | 8.43 ± 0.56 |
| 7 | Urease inhibition activity | 82.11 ± 0.14 Thiourea | 22.04 ± 0.12 Thiourea | 57.23 ± 0.50 | 375.60 ± 0.58 | 61.90 ± 0.41 | 315.00 ± 2.91 |
| 8 | Tyrosinase inhibition activity | 93.50 ± 0.14 Kojic acid | 6.04 ± 0.11 Kojic acid | 57.23 ± 0.50 | 321.60 ± 0.45 | 63.92 ± 0.41 | 305.00 ± 6.91 |
| 9 | Carbonic Anhydrase II inhibition activity | 89.03 ± 0.03 Acetazolamide | 0.10 ± 0.04 Acetazolamide | 37.43 ± 0.41 | Inactive | 73.92 ± 0.41 | 102.31 ± 0.85 |
| 10 | Phosphodiester-ase I inhibition activity | 69.02 ± 0.02 EDTA | 277.69 ± 2.52 EDTA | 1.93 ± 0.41 | Inactive | 30.92 ± 0.33 | Inactive |
Table 4. Results of biological and enzyme inhibition activities of dichloromethane and methanol extracts of whole plant of N. procumbens.
Cholinesterase inhibition activity: The acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibition activities were performed according to the method [14,15]. In both assays 60 μl KH2PO4 buffer (100 mM, pH 7.7) and 10 μl extract solution of plant (0.5 mg/ml) were mixed, followed by the addition of 10 μl enzymes. The contents were pre-incubated and pre-read. The reaction was initiated by the addition of 10 μl of DTNB. The absorbance was measured at 405 nm. Eserine was used as a standard. The results were given in Table 4.
Lipoxygenase (LOXs) inhibition activity: The reported method [16] with little modifications was used. The buffer solution of 100 mM potassium dihydrogen phosphate of pH 8 (140 μl), plant extract solution (20 μl) and 15 μl enzyme lipoxygenase (600 units/well) to made total volume of mixture (200 μl). The mixture was read at 234 nm after incubation for 10 minutes at 25°C. On addition of 25 μl solution of substrate, the reaction was initiated. The absorbance was measured after 10 minutes at 234 nm. The positive control was Baicalein (0.5 mM per well) solution. The results are given in Table 4.
Chymotripsin inhibition activity: The reported method [17] with partial modification was used in this study. The total volume of mixture (100 μl) having 50 mM Tris-HCl buffer of pH 7.6 (60 μL), 10 μL of testing plant extract solution (0.5 mg/ml) and 15 μL of enzyme (0.9 units) enzyme. After incubation for 20 minutes at 37°C, the mixture was read at 410 nm. The reaction was started by adding 15 μL of N-succinyl phenyl-alanine-p-nitroanilide substrate (1.3 mM). After half an hour, the absorbance was measured at 410 nm. The positive control was Chymostatin (0.5 mM per well). The results are given in Table 4.
Alpha glucosidase inhibition activity: The α-glucosidase inhibition activity was performed according to method [18]. The 70 μl phosphate buffer (50 mM, pH 6.8) and 10 μl of extract solution (0.5 mg/ml) were mixed, followed by the addition of 10 μl (0.057 units) enzyme. The contents were pre-incubated and pre-read. The reaction was initiated by the addition of 10 μl of p-nitrophenyl-α- D-glucopyranoside. Acarbose was used as standard. The absorbance was measured at 400 nm. The results are given in Table 4.
Urease inhibition activity: Berthelot assay has been used with partial modification [19]. The 10 μl phosphate buffer solution (pH 7.0), 10 μl of plant extract solution (0.5 mg/ml) and 25 μl of enzyme solution (0.1347 units) were added in well. The total volume of mixture was 85 μl. After incubation for five minutes, 40 μl of 20 mM urea solution was added and again incubated. After ten minutes, phenol hypochlorite reagent (115 μl/well) was added (which is fresh mixture of 45 μl phenol reagent and 70 μl of alkali reagent). On incubation at 37°C for 10 minutes, absorbance was read at 625 nm and after colour development. The results are given in Table 4.
Tyrosinase inhibition activity: In this method [20] 60 μl phosphate buffer (100 mM, pH 6.8), 10 μl plant extract solution (0.5 mg/ml) and 10 μl (5 units) of tyrosinase were mixed. The contents were pre-incubated and pre-read. After incubation, 20 μl of 10 mM L-DOPA was added. The absorbance was measured at 490 nm. Kojic acid was used as a standard. The results were given in Table 4.
Carbonic anhydrase II inhibition activity: The reported method [21] has been used for this activity. The HEPES- tris solution (140 μl) and fresh aqueous solution (20 μl) of purified bovine erythrocyte carbonic anhydrase II (0.1 mg/ ml) were mixed. The 20 μl of plant extract solution (0.5 mg/ ml) in DMSO solvent and 20 μl solution of 4-nitrophenyl acetate (0.7 mM) substrate were added. After incubation for 15 minutes at 25°C, absorbance value was taken at 400 nm. The positive control was solution of acetazolamide in this activity. The results are given in Table 4.
Phosphodiesterase I inhibition activity: In the assay [22], 97 μl of tris-(hydroxymethyl)-aminomethane buffer (50 mM), 20 μl solution of magnesium acetate (20 mM), 15 μl PDE snake venom (7.42 mg/1500 μl) were mixed. After incubation for half hour at room temperature, added 20 μl of plant extract solution (0.5 mg/ml) and reaction was started by addition of 60 μl of bis (p-nitrophenyl) phosphonate (0.33 mM) dissolved in ammonium acetate (20 mM). The absorbance was measured at 410 nm. The EDTA was used as standard. The results are given in Table 4.
Calculations and statistical analysis:
The percentage enzyme inhibition will be calculated by the following formula:
Inhibition (%)=100 - (Absorbance of test sample/ Absorbance of control) × 100.
Whereas
Absorbance of test sample=Activity in the presence of test extract
Absorbance of control=Total enzyme activity without inhibitor (extract)
IC50 values (concentration at which there is 50% in enzyme catalyzed reaction) compounds were calculated using EZ-Fit Enzyme Kinetics Software (Perrella Scientific Inc. Amherst, USA). For the determination of IC50 values, test solutions were assayed at various dilutions i.e. 0.5, 0.25, 0.125, 0.0625 mg/ml. All the results (Tables 4 and 5) are mean ± SEM (standard error of mean) of triplicate values (n=3). The statistical parameter applied is the student's t-test with p<0.05 consider as significant.
| Extract code | Type | Bacillus subtilis (G+ve) | Staphylococcus aureus (G+ve) | Pseudomonas aeruginosa, (G-ve) | Salmonella typhi (G-ve) | Escherichia coli (G-ve) |
|---|---|---|---|---|---|---|
| DCM Extract (NPD) | Inhibition (%) | 40.28 ± 0.60 | 42.89 ± 1.93 | 39.62 ± 2.45 | 40.48 ± 0.10 | 45.45 ± 3.64 |
| MIC50 | Inactive | Inactive | Inactive | Inactive | Inactive | |
| Methanol Extract (NPM) | Inhibition (%) | 48.83 ± 3.83 | 46.86 ± 2.94 | 43.68 ± 0.19 | 45.19 ± 1.00 | 45.91 ± 3.73 |
| MIC50 | Inactive | Inactive | Inactive | Inactive | Inactive | |
| Ciprofloxacin (standard) | Inhibition (%) | 91.23 ± 1.07 | 91.23 ± 1.07 | 90.88 ± 0.16 | 92.65 ± 1.10 | 91.45 ± 2.19 |
| MIC50 | 7.52 ± 0.67 | 7.03 ± 0.53 | 7.58 ± 0.19 | 7.23 ± 0.71 | 8.21 ± 0.11 |
Note: All extracts are soluble in methanol.
Table 5. Results of antibacterial activity of dichloromethane and methanol extracts of whole plant of N. procumbens.
Antibacterial activity: The antibacterial activity was performed in sterile 96-wells microplates under aseptic conditions. The method is based on the principle that microbial cell number increases as the microbial growth proceeds in a log phase of growth which results in increased absorbance of broth medium [17]. Two gram- positive bacteria (Bacillus subtilis, Staphylococcus aureus) and three gram-negative (Pseudomonas aeruginosa, Salmonella typhi, Escherichia coli) were included in the study. The microorganisms were maintained on stock culture agar medium. The extract solutions were pipette into wells (100 μg/well). Overnight maintained fresh bacterial culture after suitable dilution with fresh nutrient broth was poured into wells (180 μl). The initial absorbance of the culture was strictly maintained between 0.12-0.19 at 540 nm. The absorbance was measured at 540 nm, before and after incubation and the difference was noted as an index of bacterial growth. Ciprofloxacin was used as a standard. The results are given in Table 5.
Spasmolytic activity on isolated tissues: The animals used in the present study were local breed rabbits of both sexes, weighing 1.0-1.5 kg and maintained at 25°C in Animal House, Faculty of Pharmacy, Bahauddin Zakariya University, Multan (Pakistan). These were given fresh green fodder and tap water ad libitum. The experiments were performed in accordance with rulings of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council (NRC, 1996). The animals were subjected to fasting for 12 hours before experiments but provided free access to drinking water. The rabbits were slaughtered by stunning a blow on the back of head.
The isolated rabbit jejunum preparations were used for screening of the spasmolytic activity of the methanol extracts of N. procumbens [23]. The animals were dissected and jejunum was excised and placed in a petri dish having Tyrode's solution at 35 ± 2°C and aerated with carbogen (95% oxygen+5% carbon dioxide). The jejunum was rendered free of attached mesenteries carefully by means of sharp scissor and cut into pieces of 2 cm in length. The segments of isolated rabbit jejunum were mounted in isolated tissue organ bath filled with Tyrode's solution (10 ml) and bubbled with carbogen at 37°C. The isolated rabbit jejunum preparation was allowed to be equilibrated for 30 minutes being attached to isotonic transducer under applied tension of 0.50 mg. The spontaneous periodic contractile and relaxant activity of the mounted isolated rabbit jejunum preparations were recorded isotonically by using Power Lab Data Acquisition System.
Verapamil was used as standard drug possessing relaxant effect on spontaneous contractile activity of isolated rabbit jejunum preparation. The test plant extract (NPM) as well as standard drugs were applied to the isolated rabbit jejunum preparation in different tissue bath concentrations and response observed was used to construct graphs showing concentrations versus responses to determine the respective EC50 by using Graphpad software.
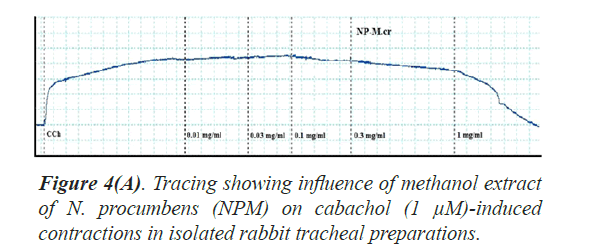
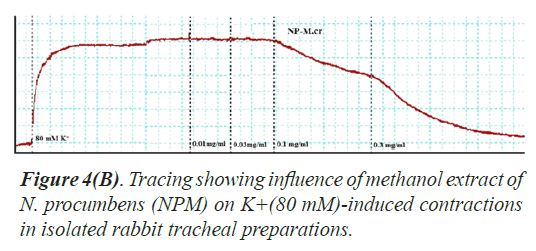
Bronchorelaxant activity on isolated tissues: The crude extract (NPM) had been screened for possible bronchorelaxant activity on isolated rabbit tracheal preparations [24]. The rabbit body was dissected and trachea was excised, cut into rings of about 2-4 mm width in a manner that each ring may contain two cartilages. The rings were opened by longitudinal cut on ventral side opposite to the smooth muscle layer. Thus, forming tracheal strips with smooth muscle sandwiched in between cartilaginous parts on the edges. The preparation was suspended in a 10 ml tissue bath containing Krebs solution at 37°C aerated with carbogen. About 1.0 gram tension was implemented to each of tracheal strip and was permitted to be equilibrated for one hour prior to recording of isometric contractions of tracheal preparation via force displacement transducers connected to power lab. The bronchorelaxant effect of the methanol extract of N. procumbens (NPM) was screened on pre-contracted isolated rabbit tracheal preparations with carbachol (1 μM) as well as K+ (80 mM). The NPM exerted relaxant effect on carbachol (1.0 μM) as well as K+(80 mM)-induced contractions in isolated rabbit tracheal preparation when applied in cumulative manner. The experiment was performed in triplicate on each preparation from 5 different animals in order to minimize animal to animal variation in results.
Results and Discussion
The dichloromethane and methanol extracts of N. procumbens were evaluated for secondary metabolites, total flavonoids and phenol contents [8-10] and presence of pharmaceutical constituents in crude extracts by using the Gas chromatography- mass spectrometry (GC-MS). Total flavonoid contents were higher in methanol extract (200.69 ± 1.97) with respect to dichloromethane extract (143.45 ± 1.25), whereas total phenol contents were 12.37 ± 0.12 only in methanol extract mg GA/g of N. procumbens extract (Table 1). The nine (09) compounds were identified in dichloromethane extract (NPD) and six (06) compounds were identified in methanol extract (NPM) of whole plant of N. procumbens (Table 2 and 3).
The total fifteen (15) compounds; (1) 3, 7, 11, 15-tetramethyl- 2-hexadecen-1-ol, (2) 12-methyl-E,E-2,13-octadecadien- 1-ol, (3) 13-heptadecyn-1-ol, (4) n-hexadecanoic acid, (5) 9,12,15-octadecatrienoic acid, 2,3-dihydroxy propyl ester, (Z,Z,Z)-, (6) Phytol, (7) Trans-13-octadecenoic acid, (8) Oleic acid, (9) Ethyl iso-allocholate, (10) Pterin-6-carboxylic acid, (11) Ergosta-5,22-dien-3-ol, acetate, (3β,22E)-, (12) 5H-cyclopropa [3,4] benz [1, 2-e]azulen-5-one, 9, 9a-bis (acetyloxy)-1, 1a, 1b, 2, 4a, 7a, 7b, 8, 9, 9a-decahydro-2, 4a, 7b-trihydroxy-3-(hydroxyl methyl)-1, 1, 6, 8-tetra methyl-, [1aR-(1aα,1bβ,2β,4aβ, 7aα, 7bα, 8α, 9β, 9aα]-, (13) Hexadecanoic acid, methyl ester, (14) 7,10-Octadecadienoic acid and (15) 10-Octadecenoic acid, methyl ester were identified in whole plant material extracts (NPD and NPM) of Neurada procumbens by performing the GC-MS analysis.
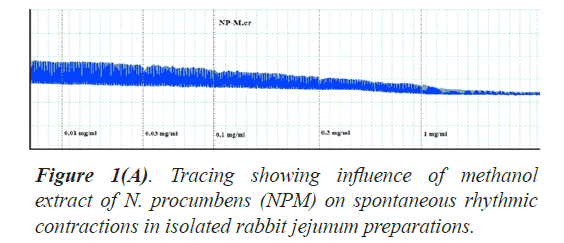
The twelve (12) different kinds of in vitro biological and enzyme inhibition activities antioxidant, anticholinesterase, antilipooxygenase, antichymotripsin, antialpha glucosidase, antiurease, antityrosinase, anticarbonic anhydrase II, antiphosphodiesterase I, antibacterial, spasmolytic and brochorelaxant activities were performed including the two activities (spasmolytic and bronchorelaxant activities) on isolated tissues to evaluate the diverse folkloric use of the plant N. procumbens scientifically (Table 4). The dichloromethane extracts (NPD) of N. procumbens showed antilipooxygenase, antichymotripsin, antialpha glucosidase, antiurease and antityrosinase activities. The methanol extracts (NPM) of N. procumbens showed antioxidant, antichymotripsin, antialpha glucosidase, antiurease, antityrosinase, anticarbonic anhydrase II, spasmolytic and brochorelaxant activities. Spasmolytic activity of methanol extract (NPM) on rabbit jejunum was demonstrated (Figures 1A and 1B) caused concentration dependant inhibition of spontaneous contractions (0.01-1.0 mg/ml) with EC50 value 0.157 μM.
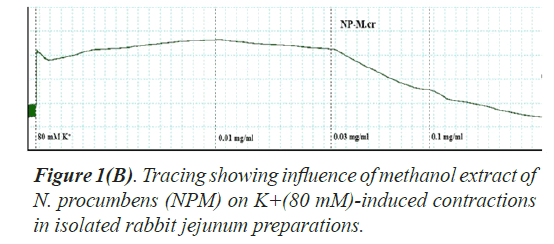
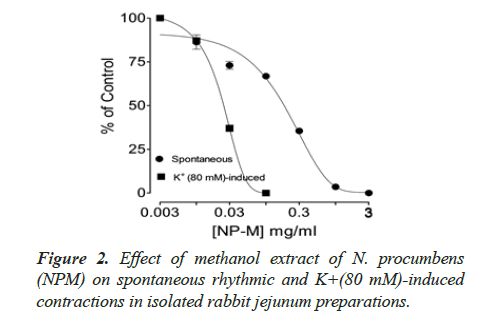
Spontaneous and K+(80 mM) induced contractions (0.01-0.1 mg/ml) with EC50 value 0.024 μM. Spontaneous and K+(80 mM) induced contractions were completely inhibited by standard drug verapamil (Figures 2 and 3).
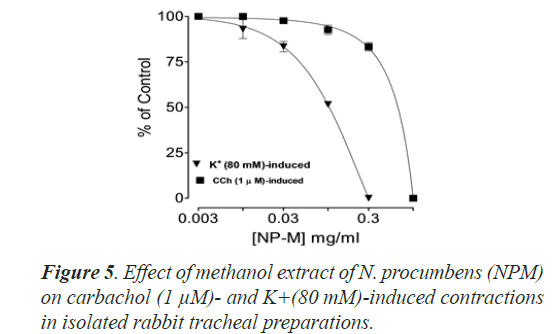
Bronchorelaxant activity of methanol extract (NPM) on rabbit trachea, caused relaxation for carbachol (1.0 μM) between 0.011-0.3 mg/ml with EC50 value 0.624 μM and high K+ (80 mM) induced contractions (0.01-0.1 mg/ml) with EC50 value 0.096 μM (Figures 4 and 5). The isometric contractile response was recorded through Power lab Data Acquisition system (AD instruments, Sydney, Australia) attached to a computer system having software Lab Chart (version 7). The standard drug Verapamil having Ca++ channel blocking effect was tested on carbachol (1.0 μM) and K+(80 mM) induced spastic contractions to evaluate the spasmolytic and bronchorelaxant effect of methanol extract (NPM) of N. procumbens. N. procumbens has folkloric value in the management of multiple diseases pertaining to hyperglycemia (diabetes), gastrointestinal (diarrhea, dysentery, ulcer), cardiac (antioxidant use) and respiratory (asthma, inflammation) systems. The present work was a scientific validation of folkloric claims of the plant.
On phytochemical screening, it was found that N. procumbens is rich source of flavonoid contents (Table 1). The fifteen (15) compounds were identified in the plant first time by using the GC-MS technique (Tables 2 and 3). The only methanol extract (NPM) possess very good antioxidant activity with inhibition 75.38 ± 3.16% with IC50 88.30 ± 1.21 μMoles which is significant comparable to standard quercetin (83.68 ± 3.76% with IC50 16.96 ± 0.14 μMoles) proving the antioxidant potential of the plant in oxidative stress and cardiac problems [25-27]. The products of lipoxygenase play a vital role in many medical disorders such as inflammation, bronchial asthma [29] and tumor angiogenesis [30]. The only dichloromethane extract NPD possess moderate lipoxygenase inhibition activity with value 44.60 ± 0.23% with IC50 524.60 ± 1.02 μMoles in comparison to standard Baicalein (93.79 ± 1.27% with IC50 22.47 ± 0.04 μMoles), which linking the importance of the plant in bronchial problems. The dichloromethane extract exhibited 84.38 ± 0.05% inhibition of chymotrypsin with IC50 value 115.71 ± 0.03 μM which is significant and close to value of standard chymostatin (93.50 ± 0.91%) which indicates that non-polar or less polar compounds present in this extract are responsible for this activity and proving the folkloric importance of plant in the treatment of edema and inflammation [31-33].
The alpha glucosidase enzyme catalysis the hydrolysis of disaccharides into glucose, inhibition of this enzyme can suppress the post prandial hyperglycemia and this inhibition will be useful invention for management of diabetes type II [36]. The alpha glucosidase inhibition activity of dichloromethane and methanol extracts of N. procumbens was significant and higher than acarbose. The dichloromethane extract possess percentage inhibition 95.49 ± 0.65 with IC50 24.32 ± 0.62 μMoles which was more potent than standard inhibitor acarbose (92.23 ± 0.14% with IC50 38.25 ± 0.12 μMoles) whereas the percentage inhibition and IC50 values of methanol extract (99.45 ± 0.58 with IC50 8.43 ± 0.56 μMoles) was found five times more potent than the standard acarbose. Urease is of medical importance because it is involved for establishing disease state like peptic ulcer, kidney stones, pyelonephritis and other diseases [30]. So due to the role of urease in the production of certain diseases it is of much importance in pharmaceutical research [28]. Urease enzyme is found in most of the organisms. It breaks down the urea to ammonium carbonate and is mostly used for determination of urea [33]. Urea catalysis is carried out by urease forming carbonate anions and ammonium cations. Research is going on urea in many laboratories as it has gained much worth in biotechnology and medicine [34]. The both NPD and NPM extracts possess urease inhibition activity with values 57.23 ± 0.50% with IC50 375.60 ± 0.58 μMoles and 61.90 ± 0.41% with IC50 315.00 ± 2.91 μMoles respectively, whereas the standard thiourea (82.11 ± 0.14% with IC50 22.04 ± 0.12 μMoles) was used which indicates the non-polar or less polar compounds present in the plant producing this activity.
Tyrosinase over production causes hyperpigmentation and ocular retinitis pigmentosa. Hyperpigmentation in human skin makes the researcher to findout a potent tyrosinase inhibitor as skin whitening agent [37]. This enzyme is also related to the formation of neuromelanin in human brain which leads to neurodegeneration [25]. Keeping in view this importance, tyrosinase inhibition activity was evaluated and found 57.23 ± 0.50% and 63.92 ± 0.41% for dichloromethane extract (NPD) and methanol extract (NPM), respectively against the standard Kojic acid (93.50 ± 0.14%). The results are given in Table 4. The antibacterial activity of both dichloromethane and methanol extracts was evaluated against two gram-positive bacteria (Bacillus subtilis, Staphylococcus aureus) and three gram-negative (Pseudomonas aeruginosa, Salmonella typhi, Escherichia coli). The results are given in Table 5 shows that bacterial growth inhibition is ranging from 39.62 ± 2.45 to 48.83 ± 3.83%, which is not significant and inhibition was less than 50% against all strains.
The folkloric uses in gastrointestinal and respiratory systems of the N. procumbens was evaluated by searching spasmolytic and bronchorelaxant activities by observing the effect of methanol extract of the plant (NPM) on isolated tissues of rabbit jejunum and trachea. Spasmolytic activity on rabbit jejunum was established using methanol extract of N. procumbens. The concentration-dependent inhibition of spontaneous contractions (0.01-1 mg/ml) with EC50 value 0.157 μM and K+ (80 mM) induced contractions (0.01-0.1 mg/ ml) with EC50 value 0.024 μM were observed. Spontaneous K+ (80 mM) induced contractions were completely inhibited by standard drug verapamil (Figures 1A and 1B).
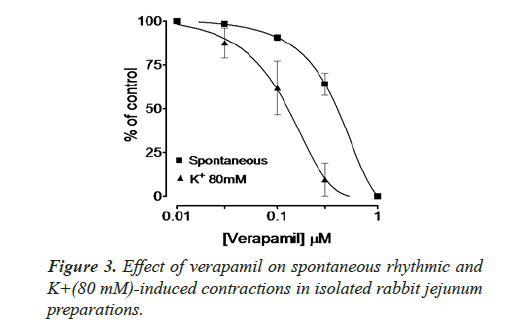
Inhibitory effect of crude methanol extract of N. procumbens was observed on spontaneous contraction of rabbit jejunum that suggests a spasmolytic activity similar to verapamil (Figures 2 and 3). Verapamil was used as standard exhibited relaxation effect on spontaneous contracting jejunum in dose range of 0.03-1.0 μM with EC50 value 0.35 μM and K+ (80 mM) in dose range 0.03-0.3 μM with EC50 value 0.13 μM (Figure 3). The possible mechanism was investigated by using a high K+ (80 mM) was used to cause smooth muscles contractions through opening of calcium channels which results in increase of intra cellular calcium and contractile effect [7]. This result supports the traditional use of N. procumbens in hyperactive gut or in disease diarrhea as calcium antagonist [35-38].
Justification for the folk use of N. procumbens in respiratory disorders was obtained by experiments on the isolated rabbit trachea. The Figures 4A, 4B and 5 show that methanol extract of N. procumbens relaxed tracheal preparation pre contracted with carbachol (1.0 μM) or K+ 80 mM in a concentration dependent fashion similar to standard verapamil. It indicates non-specific bronchodilation of trachea mediated through calcium channel blockage. When methanol extract of N. procumbens was applied against carbachol (1.0 μM) and high K+ (80 mM), it produced bronchodilator effect on rabbit trachea and concentration dependent inhibition with respective EC50 value of 0.624 mg/ml (0.01-0.3 mg/ml) and value of EC50 was 0.096 mg/ml (0.01-0.1 mg/ml) (Figures 4A and 4B). In our previous work, we have evaluated and proved the spasmolytic effect of another medicinal plant Farsetia hamiltonii Royle from the same source Cholistan desert of Pakistan [38].
Conclusion
The results of antioxidant, antilipoxygenase, antichymotrypsin, anti-alpha glucosidase, antiurease, antityrosinase, anticarbonic anhydrase II, spasmolytic and brochorelaxant activities obtained from this study may provide the scientific evidence for folkloric uses of Neurada procumbens in treating oxidative stress, asthma, inflammation, edema, diabetes, skin, gastrointestinal and respiratory disorders. So, this study offers rational hypotheses for antidiarrheal, antiasthmatic and antidiabetic medicinal uses of N. procumbens L. Further, work is required regarding evaluation of medicinal effects of fifteen (15) identified compounds from this study to find out the molecule, which may make basis of the development of a new drug in gastrointestinal, respiratory ad hyperglycemic disorders.
Acknowledgment
The authors are grateful to the PhD scholar Mr. Zafar Mahmood, Department of Statistics, The Islamia University of Bahawalpur, Bahawalpur, Pakistan on providing facilities and help in statistical calculations.
Author’s contribution
It is declared that this work was done by first author under the supervision of second author named in this article and all liabilities pertaining to claims relating to the content of this article will be borne by them.
References
- Abdelaaty AS, Mansour SA, Muhammad AA, Maureen H, Albena T, Dinkova-Kostova. NAD (P) H: Quinone oxidoreductase 1 inducer activity of some Saudi Arabian medicinal plants. Planta Med 2013; 79: 459-464.
- Ahmed N, Mahmood A, Mahmood A, Tahir SS, Bano A, Malik NR, Hassan S, Ishtiaq M. Relative importance of indigenous medicinal plants from Layyah district, Punjab Province Province, Pakistan. J Ethnopharm 2014; 155: 509-523.
- Ahmed N, Mahmood A, Tahir SS, Bano A, Malik NR, Hassan S, Ashraf A. Ethanomedicinal knowledge and relative importance of indigenous medicinal plants of Cholistan desert, Punjab Province, Pakistan. J Ethnopharm 2014; 155: 1263-1275.
- Arslan O. Inhibition of bovine carbonic anhydrase by new sulfonamide compounds. Biochem (Mosc) 2001; 66: 982-983.
- Atta UR, Iqbal M, William J. Bioassay techniques for drug development. Harwood Academic Publishers 2001; 14-25.
- Baylac S, Racine P. Inhibition of 5-lipoxygenase by essential oils and other natural fragrant extracts. Int J Aromather 2003; 13: 138-142.
- Bolton TB. Mechanism of action of transmitter and other substances on smooth muscles. Physiol Rev 1979; 59: 606-718.
- Brain KR. The practical evalution of phytochemicals, Wright-Scientechnica, Bristol 1975; 152-153.
- Chen HB, Islam MW, Radhakrishnan R, Wahab SA, Naji MA. Influence of aqueous extract from Neurada procumbens L on blood pressure of rats. J Ethonopharm 2004; 90: 191-94.
- MI, Adhikari A, Rasheed S, Marasini BP, Hussain N, Kaleem WA, Atta-ur- Rehman. Cyclopeptide alkaloids of Ziziphus oxyphylla Edgw as novel inhibitors of α-glucosidase enzyme and protein glycation. Phytochem letters 2001; 4: 404-406.
- David Sparkman O, Zelda P, Fulton GK. Gas Chromatography and Mass Spectrometry: A Practical Guide 2011.
- Hites and Ronald A. Development of gas chromatographic mass spectrometry. Anal Chem 2016; 88: 6955-6961.
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid calorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7: 88-95.
- Fishbein W, Gutwein BM. Urease catalysis and structure. J Bio Chem 1973; 248: 870-877.
- Gilani AH, Atta-ur-Rahman. Trends in Ethanopharmacology. J Ethanopharm 2005; 100: 43-49.
- Gilani AH, Aziz N, Khurram I, Rao ZA, Ali BA. The presence of cholinomimetric and calcium antagonist constituents in Piper betle Linn. Phytother Res 2000; 14: 338-344.
- Gilani AH, Shaheen F, Zaman M, Janbaz KH, Shah BH, Akhtar MS. Hypotensive and Spasmolytic activities of crude extract of Cyperus scariosus. Arch Pharmacol Res 1999; 17: 145-149.
- Hayat MM, Sarwar S, Anjum S, Uzair M, Rasheed HMF, Jabeen Q, Choudhary BA, Ashraf M. Anti-diabetic and spasmolytic potential of Farsetia hamiltonii Royle from Cholistan desert. J Ethnopharm 2014; 156, 347-352.
- Ismail A, Marjan ZM, Foong CW. Total antioxidant activity and phenolic content in selected vegetables. Food Chem 2004; 87: 581-586.
- Khalid MK, Naheed F, Maimona R, Saima J, Nida A, Shahnaz P, Choudhary MI. 1,3,4-Oxadiazole-2(3H)-thione and its analogues: A new class of non-competative nucleotide pyrophosphatases/phosphodiesterases 1 inhibitors. Bioorg Med Chem 2009; 17: 7816-7822.
- Kim H, Lee SH, Choi SY, Hwang JS, Lee BG, Gao JJ, Kim SY. Mulberroside F isolated from the leaves of Morus alba inhibits melanin biosynthesis. Bio Pharm Bulletin 2002; 25: 1045-1048.
- Kim JS, Kwon CS, Son KH. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Bioscience, Biotechnol Biochem 2000; 64: 2458-2461.
- Kubo I, Chen QX, Nihei K, Calderón JS, Céspedes CL. Tyrosinase inhibition kinetics of anisic acid. Z Naturforsch C 2003; 58: 713-718.
- Lee SE, Hwang HJ, Ha JS, Jeong HS, Kim JH. Screening of medicinal plant extracts for antioxidants activity. Life Sci 2003; 73: 167-179.
- Lois MB, Ronald LS. Application of the Berthelot reaction for rapid detection of urea hydrolysis by proteus strains. J Bacteriol 1963; 85: 954-955.
- Marzouk MM, Hussein SR, Ibrahim LF, Elkhateeb A, Kawashty SA, Saleh NAM. Flavonoids from Neurada procumbens L. (Neuradacea) in Egypt. Biochem Syst Ecol 2004; 57: 67-68.
- Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS, Leitao SG. Screening of Brazilian plant extracts for antioxidants activity by use of DPPH free radical method. Phytother Res 2001; 15:127-130.
- Mobely HL, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiol Mol Biol Rev 1995; 59: 451-480.
- Mossa JS, Al-Yahya MA, Al-Meshal IA, Tariq M. Phytochemical and biological screening of Saudi plants-part 5. Fitoterapia 1983; 149-161.
- Mossa JS, Al-Yahya MA, Al-Meshal IA, Tariq M. Phytochemical and biological screening of Saudi plants-part 4. Fitoterapia 1983; 75-80.
- Nasir E, Ali SI. The Flora of West Pakistan, An annotated catalogue of the vascular plants of West Pakistan and Kashmir. Fakhri Printing Pre 1972; 353.
- Parvez S, Kang M, Chung HS, Bae H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother Res 2007; 21: 805-816.
- Rahmatullah Q, Bhatti GR, Rabia AM. Ethnomedicinal uses of herbs from northern part of nara desert, Pakistan. Pak J Bot 2010; 42: 839-851.
- Robinson W, Wilson CS. Changes in concentration of urease during pupal development of the Blowfly. J Parasitol 1939; 25: 455-458.
- Sharkey KA and Wallace JL. Treatment of disorders of bowel motility and water flux. Antiemetics, agents used in biliary and pancreatic diseases. Pharmacol Basis Thera 2011; 12: 1223-1349.
- Steinhilber D. A target for anti-inflammatory drugs revisited. Current Med Chem 1999; 6: 71-85.
- Tappel AL. The mechanism of the oxidation of unsaturated fatty acid catalyzed by hematin compounds. Arch Biochem Biophys 1953; 44: 378-395.
- Tarun EE, Zheldakova TA, Metelitza DI. Substituted 1,5,6,7-tetrahydro-4-benzimidazole-4-ones as urease inhibitor. Biochem 2009; 3: 71-76.
- Vinha AF, Soares MO, Castro A, Santos A, Oliveira MBPP, Machado M. Phytochemical characterization and radical scavenging activity of aqueous extracts of medicinal plants from Portugal. Eur J Med Plants 2012; 2: 335-347.
- Zengin G, Cakmak YS, Guler GO, Aktumsek A. In vitro antioxidant capacities and fatty acid compositions of three Centaurea species collected from Central Anatolian region of Turkey. Food Chem Toxicol 2010; 48: 2638-2641.