ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2009) Volume 20, Issue 2
Glycation of lens crystalline protein in the pathogenesis of various forms of cataract
Ashok V. Kattaa, A.N. Suryakarb, R.V. Katkamc, Kayyum Shaikha, Santoshi R. Ghodakea
aDepartment of Biochemistry, K. B. N. Institute of Medical Sciences, Gulbarga, Karnataka, India
bDepartment of Biochemistry Shri. R. C. S. M. Govt. Medical College CPR Hospital, Kolhapur. Maharashtra
cDepartment of Biochemistry, Dr. V. M. Govt. Medical College, Solapur, Maharashtra, India
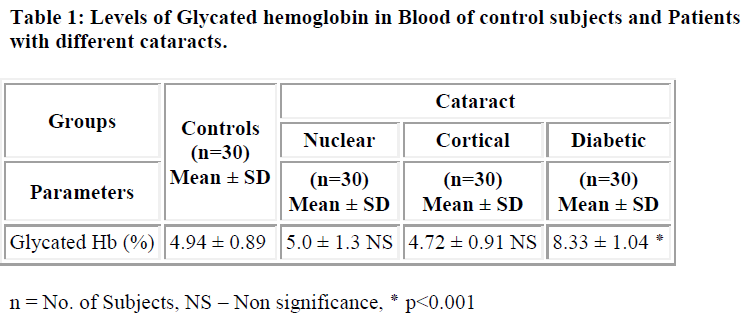
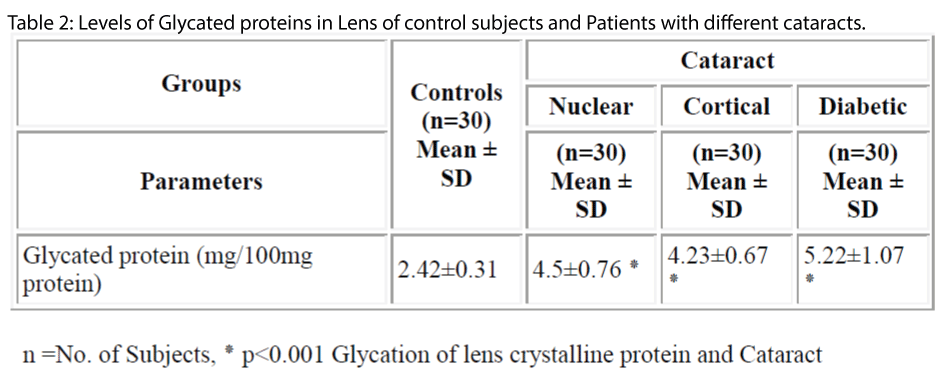
The pathogenesis of cataract has been found to be influenced by number of factors including protein glycation. Cataract is the clouding or opacity that develops in the lens of eye. It forms when bounding or folding of protein changes and clump together; eventually these clumps cloud the lens and block the light. The aim of this study was to estimate the levels of glycated protein in blood and lens in patients with cataract. The levels of protein glycation both in serum and lens of controls and different type of cataract were studied. The levels of glycation of lens protein was significantly higher in both age related cataract (4.5±0.76 mg/100mg of protein) and diabetic cataract (5.22±1.07mg/100mg of protein) (p<0.001) when compared controls (2.42±0.3107mg/100mg of protein). Similarly the levels of glycated hemoglobin in diabetic cataract (8.33 ±1.04%) was significantly higher (p<0.001) when compared with controls (4.94±0.89 %). The levels of protein glycation were significantly higher in diabetic cataract (5.22±1.07mg/100mg of protein) (p<0.001) when compared with other types of cataract (nuclear cataract 4.5± 0.76mg/100mg of protein, cortical cataract 4.23±0.67mg/100mg of protein). These results show that the increase in glycation process may accelerates the development of cataract in diabetic patients.
Keywords
Age related cataract, Diabetic Cataract, Lens, Protein Glycation, Glycated hemoglobin.
Introduction
Amongst five senses, vision is the most evolved sense in the human being. Cataract is the clouding or opacity that develops in the eye [1]. Cataract is a leading cause of visual disability and blindness throughout the world [2].
Cataract that develops in older people without a known cause is called Senile cataract [3]. Where as diabetes is considered as major risk factor for the development of „senile-type? cataract [4]. Evidence suggests that glycation of lens proteins is one of the cause of cataract formation [5-7].
Glycation of lens protein is the process in which glucose or the reducing sugars reacts with e-amino group of lysine resulting in the Schiff base (SB) formation. This SB undergoes an Amadori rearrangement via Maillard reaction to form Amadori product. Later, the Amadori product undergoes dehydration and rearrangement to form cross-links of proteins. Resulting in protein aggregation or advanced glycation end products (AGEs)
Given the extent of disability caused by cataract it is important that some measures are to be taken to slow down the development of the cataract, as we cannot prevent it from occurring. A delay in cataract formation for 10 years will reduce the prevalence of cataract by 50%, such delay will enhance the quality of the life for much of the older population and reduces the economic burden due to visual disability and surgery [9].
So the present study was undertaken to evaluate and compare the extend of protein glycation in various forms of cataracts.
Materials and Methods
The present study was carried out in the Department of Biochemistry, Dr. V.M. Government Medical College, Solapur. The patients were selected from those who were admitted for cataract extraction in the Department of Ophthalmology, S. C. S. M. General Hospital, Solapur.
The study was conducted on the lens and sera of 120 individuals between age group of 50 to 80 years who were distributed in two groups viz. study group and control subjects. Study group includes 90 cataract patients. This study group was further divided in Nuclear cataract, Cortical Cataract & Diabetic cataract of 30 patients each. Control group comprised of 30 persons with visual activity of 6/6 or better in both the eyes and to whom antioxidant medicines were not given. They were all healthy individuals without any systemic diseases and without any habits like smoking, alcoholism, etc. Patients with ocular surgery, trauma, infection, inflammation of the eye were also excluded from the study.
Collection of lenses and preparation of homogenate3
The cataractous lenses were removed surgically by PECCE + IOL technique. The lenses were obtained and packed in ice-cold saline and immediately taken to the laboratory where the analyses were performed.
Each lens was weighed and its wet weight (mg) recorded. The lenses were homogenized with a Teflon rod and diluted to 1:10 (w/v) by adding 50mM potassium phosphate buffer (2mM EDTA), PH 7.00. The lens homogenates obtained were centrifuged at 10,000 rpm for 15 min at 4°C. The supernatants obtained following centrifugation were processed for estimation of biochemical parameters [10].
Blood collection
The 3ml blood samples were collected in a heparinized bulb by venipuncture from the patients undergoing surgery for cataract on the same day for the assessment of Glycated hemoglobin. The results were expressed in mean ± SD. One way analysis of variance (ANOVA) was used to test the significance of difference and student “t” test to test significance of difference between two groups.
Extent of protein glycation was measured by the method of Fluckger and Winterhalter [11] improved by Eross. In this method glycated protein is quantitatively hydrolyzed by heating in presence of acid. Resultant chromogen is measured colorimetrically as adduct of thiobarbituric acid at 448 nm.
Results and Discussion
The levels of glycosylated hemoglobin in case of diabetic cataract (8.33 ± 1.04%) were found to be significantly higher (p<0.001) than in controls (4.94 ± 0.89%) (Table-1). In case of lens, the levels of glycated protein were found to be significantly higher (p<0.001) in diabetic cataract (5.22±1.07mg/100mg of protein) followed by nuclear (4.5±0.76 mg/100mg of protein) and cortical cataract (4.23±0.67 mg/100mg of protein) when compared with controls (2.42±0.31 mg/100mg of protein) (Table-2). These results are consistent with earlier studies showing that there are only slight differences in glycation of lens proteins in nuclear and cortical cataract patients[4,12] and that glycation of lens proteins is constant with age [13,14].
These results might be due to the fact that in diabetes, permeability of glucose from the plasma to aqueous and vitreous compartments is abnormally high. Also, damage to the ocular vasculature has been identified as on early abnormality in diabetes. This change in the permeability barriers to glucose in diabetes could amplify the effects of hyperglycemia on the lens [4].
Another mechanism proposed by Stevens and Monnier hypothesized that non-enzymatic glycosylation might enhance the susceptibility of lens to sulphydryl oxidation and high molecular weight protein aggregation. Oxidative damage of protein is one of the modification leads severe failure of biological function and death. Since the lens proteins are long-lived, they are highly susceptible to post-translational modification such as glycation which is believed to enhance protein unfolding, changing not only the physiochemical properties of lens proteins but also its function [15].
Conclusion
Breakdown in the lens-gulcose barrier followed by increased lens protein glycation, nonenzymatic browning may contribute to the initiation and acceleration of cataractogenesis in diabetes. Further studies, particularly on the concentration of oxidative products of lens crystalline in cataract may clarify the role of oxidation in the development of senile and diabetic cataract.
References
- Hualei Li. Free radicals and cataract. Spring 2003; 222(77): 1-23.
- Zehra Hashim; Shamshad Zarina. Antioxidant markers in human senile and diabetic cataractous lenses. JCPSP 2006; 16(10): 637-640.
- Sabu George, Jyothi M, Betzy Mathew, S. Shashidhar. Changes in Glutathione, Glutathione- linked enzymes and hexose monophosphate shunt enzymes in senile cataract. Indian J Physiol Pharmacol 2003; 47(2): 191-196.
- Timothy J. Lyons; Giuliana Silvestri; John A. Dunn; Daniel G. Dyer. Role of glycation in modification of lens crystallins in diabetic and nondiabetic senile cataract. Diabetes 1991; 40: 1010-1015.
- Lewis B. S. and Harding J. J. The effect of aminoguanidine on the glycation (non- enzymatic glycosylation) of lens protein. Exp. Eye.Res. 1990; 50: 463-467.
- Cherian M. and Abraham E. C. Glycation of Human Lens Crystallins effect of age and aspirine treatment. Ophthalmic Res. 1993; 25: 349-354.
- Cherian M. and Abraham E. C. In vitro glycation and acetylation (by aspirin) of rat crystallins. Life Sci. 1993; 52: 1699-1707
- Jansirani; Anathanaryanan P.H. A comparative study of lens protein glycation in various forms of cataract. Indian Journal of Clinical Biochemistry 2004; 19(1): 110-112.
- Balasubramanian D.; Aashish K.Bansal; Surendra Basti. et al. The biology of cataract. Ind. J. Ophthalmology. 1993; 41(4): 153-171.
- Donma Orkide; Eda Ozkanat Yorulmaz; Hamiyet Pekel; Nezir Suyugul. Blood and lens lipid peroxidation and antioxidant status in normal individuals, senile and diabetic cataractous patients. Cur. Eye Res. 2002; 25(1): 9-16.
- Fluckiger Winterhalter. Glycated Hemoglobin (GHb). In: Clinical Chemistry, Principle and Practice by Praful Godkar. 1994; 1st Edi, 115-116.
- Oimomi M., Maeda Y, Hata F, Kitasumoto S., et al. Glycation of cataractous Lens in non-diabetic senile subjects and diabetic patients. Exp. Eye. Res. 1988; 46:415-420.
- Dunn J A, Patrick JS, Thorpe SR, Baynes JW. Oxidation of Glycated protein: age-dependent accumulation of N (carboxymethyl)lysine in lens proteins. Biochemistry 1989; 28: 9464-9468.
- Patrick JS, Thorpe SR, Baynes JW. Non-enzymatic glycosylation of protein does not increase with age in normal human lenses. J Gerontol Biol Sci. 1990; 45: B18- 23.
- Stevans, V.J.; Monnier, V.M.; Ceramin. Diabetic Cataract Formation: Potential role of Crystallins glycosylation of lens. Proc. Natl. Acad. Sci. USA 2002; 75: 2918-2922.