ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 10
GON4L drives nasopharyngeal carcinoma growth and proliferation through regulation of β-catenin/wnt singling pathway
Fei Xia1#, Peng Jin1#*, Yanan Ding2, Feng Li1 and Chao Shi1
1Department of Otorhinolaryngology, Xiangyang No.1 People’s Hospital Affiliated to Hubei University of Medicine, Hubei, P. R. China
2Department of Anesthesiology, Xiangyang No.1 People’s Hospital Affiliated to Hubei University of Medicine, Hubei, P. R. China
#These authors contributed equally to this work
- *Corresponding Author:
- Peng Jin
Department of Otorhinolaryngology
Xiangyang No.1 People’s Hospital Affiliated to Hubei University of Medicine
P. R. China
Accepted date: February 17, 2017
Nasopharyngeal Carcinoma (NPC) is the most common cancer originating in the nasopharynx. Aberrant proliferative signals and apoptotic dysregulation are required for NPC development. However, the mechanisms behind them are still unclear. In this study, we explored role of GON4L, a new-found transcriptional repressor, in NPC cells. Knockdown of GON4L expression impaired NPC cells colony formation and proliferation. Cisplatin-induced apoptosis was enhanced by downregulation of GON4L. Forced expression of GON4L resulted in promoted proliferation and inhibited apoptosis of NPC cells induced by Cisplatin. Furthermore, GON4L knockdown inhibited β-catenin accumulation, wnt, P53 and Bcl-2 expression. Inhibition of GON4L transcriptional suppressive activity reversed GON4L-induced NPC cells proliferation and survival. In conclusion, these data suggest that oncogenic role of GON4L and provide a new potential therapeutic target for NPC treatment.
Keywords
Nasopharyngeal carcinoma, GON4L, Apoptosis, Proliferation, β-catenin/wnt
Introduction
Nasopharyngeal Carcinoma (NPC) is one kind of malignant tumors characterized by higher invasiveness, metastatic potential in the world [1,2]. It is a high incidence of malignant tumor in China and presents higher mortality in clinical [3-5]. Clinical observation and experimental study indicate pathogenic factors of nasopharyngeal carcinoma are extensive, such as hereditary factors, virus infections, and environmental factors [6,7]. Evidences have revealed that NPC initiates from the epithelial cell of nasopharynx and is famous for its regional lymph node metastasis and distant metastasis in NPC patients [8,9]. Strategies of inhibition of migration and invasion have been proposed in clinical regiment for NPC therapy [10]. Understanding mechanism(s) of these local metastasis and distant metastasis are essential for the progression of NPC patients.
GON4L is a new identified transcriptional repressor. Genetic study has indicated that GON4L plays essential role in haematopoiesis by regulation of YY1, co-repressor Sin3a, and histone deacetylase 1 in zebrafish and mice experiments [11]. In recent year, GON4L is identified as a transcriptional repressor in human cancer cells, which can stimulate cancer growth through an YY1-androgen receptor-CD24 axis [12]. Lu et al. have indicated that Gon4l protein is required for B lymphopoiesis and may function to regulate gene expression during these molecular processes [13]. Therefore, we assumed that GON4L may be a potential target for NPC therapy.
Previous studies have revealed that β-catenin/wnt signal pathway involves in the progression of growth and aggressiveness of human cancer [14,15]. Weinberger et al. have showed that association of nuclear, cytoplasmic expression of galectin-3 with beta-catenin/Wnt-pathway activates thyroid carcinoma activity and stimulates cells cycle of thyroid carcinoma cells [16]. Interestingly, β-catenin/wnt signalling pathway is known to have a critical role in carcinogenesis and in epithelial-to-mesenchymal transition in fibromatosis, metaplastic carcinomas and phyllodes tumours of the breast [17]. In this study, we presumed GON4L regulates nasopharyngeal carcinoma growth and proliferation through regulation of β-catenin/wnt singling pathway. Here, we present evidences that GON4L knockdown significantly inhibits growth and aggressiveness of NPC cells through down-regulation of β-catenin/wnt signaling pathway. Importantly, apoptosis of nasopharyngeal carcinoma cells was markedly promoted by GON4L knockdown.
Materials and Methods
Cells and reagents
Nasopharyngeal carcinoma cell lines HNE1 and CNE2 were purchased from American Type Culture Collection (ATCC). All tumor cells were cultured in MEM (Sigma-Aldrich) medium (Gibco, CA, USA) supplemented with 10% Fetal Calf Serum (FCS) (Invitrogen, CA, USA). All cells were cultured in a 37°C humidified atmosphere of 5 % CO2.
Endogenous overexpression of GON4L
HNE1 and CNE2 cells were cultured until 85% confluence and the media was then removed from cultural system. Cells were transfected by pedue12.4-GON4L using lipfectamine 2000 (Sigma-Aldrich). Stable ERK-overexpression HNE1 and CNE2 cells were selected by GS screening system [18].
Transfection of small interference RNA (Si-RNA)
All siRNAs were synthesized by Invitrogen (Shanghai, China) including Si-RNA-GON4L (Si-FasL) or Si-RNA-vector (Si- Vector). HNE1 and CNE2 cells (1 × 106) were transfected with 100 pmol of Si-GON4L (Applied Biosystems) with Si-vector as control (Applied Biosystems) by using a Cell Line Nucleofector kit L (Lonza).
MTT assays
HNE1 and CNE2 were transfected by Si-vector or Si-GON4L and culture in 96-well plates for 48 h. After incubation, 20 μl of MTT (5 mg/ml) in PBS solution was added to each well, the plate was further incubated for 4 h. Most of the medium was removed and 100 μl of DMSO was added into the wells to solubilize the crystals. The OD was measured by a BIO-RAD (ELISA) reader at wavelength of 450 nm.
RNA isolation and quantitative RT-PCR (qRT-PCR)
Total RNA from HNE1 and CNE2 cells was extracted using RNAeasy Mini Kit (QIAGEN, Gaithersburg, MD). All the forward and reverse primers were synthesized by Invitrogen. All mRNA levels were quantified by using Power SYBR Green Master Mix (Applied Biosystem). Relative mRNA expression levels were calculated by 2−ΔΔCt. The results analysed in triplicate according to the Delta-Delta threshold (ΔΔCt) method as the n-fold way compared to control.
Tumor cell colony formation assay
HNE1 and CNE2 cells after knockdown or overexpression of GON4L (1 × 104) were plated in six-well plates. Cells proliferation were stained with crystal violet (0.005%) for 30 minutes and photographed. Clonal numbers of HNE1 and CNE2 cells were analysed by Alpha Innotech (San Leandro, CA) imaging software.
Cell invasion and migration assays
For invasion assay, HNE1 and CNE2 cells after knockdown or overexpression of GON4L cells were suspended as a density of 1 × 106 in 500 μl in serum-free MEM. HNE1 and CNE2 cells were subjected to the tops of BD BioCoat Matrigel Invasion Chambers (BD Biosciences) according to the manufacturer’s instructions. For migration assay, HNE1 and CNE2 cells were subjected to a control insert (BD Biosciences) instead of a Matrigel Invasion Chamber. The tumor cells migration and invasion were counted in at least three randomly stain-field microscope every membrane.
Apoptosis assay
Apoptosis of HNE1 and CNE2 cells was assessed by incubation these cells with Cisplatin (3.0 mg/ml) for 48 h. After incubation, HNE1 and CNE2 cells were trypsinized, collected, washed in cold PBS, and adjusted to 1 × 106 cells/ml with PBS, labeled with annexin V-FITC and PI (Annexin VFITC Kit, BD), and analysed with a FACScan flow cytometer (BD).
Western blotting
The primary antibodies used in the immunoblotting assays were: GON4L (ab74049, Abcam), caspase-3 (ab2172, Abcam), caspase-12 (ab62484, Abcam), Bcl-2 (ab692, Abcam), P53 (ab61241, Abcam), β-catenin (ab32572, Abcam), wnt (ab15251, Abcam), and β-actin (ab8826, Abcam). Horseradish peroxidase-conjugated secondary antibody (Bio-Rad, Hercules, CA, USA) was used at a 1:5,000 dilution and detected using a Western Blotting Luminol Reagent.
Immunofluorescence
HNE1 and CNE2 cells were fixed were fixed with paraformaldehyde (4%) in HEPES buffer (50 mM, pH 7.3) for 1 hour and permeabilized with 0.2% Triton X-100 in PBS (pH 7.4) for 30 minutes at room temperature. The cells were then incubated with the rabbit anti-human primary antibodies (Survivin (ab469) and LIMD2α (ab205375) diluted 1:50 in T-PBS. Samples were settled at 4°C overnight and incubated further with secondary antibodies Alexa Fluor 488-conjugated goat anti-rabbit IgG and Alexa Fluor 594 phalloidin (catalog NO A12381, Life Technologies), respectively. All samples were cover slipped with Dako fluorescence mounting medium (catalog NO S3023, Dako, Carpentaria, CA). Impaired neurogenesis was analysed by fluorescence microscope (Olympus BX61, Japanese).
Statistical analysis
Results are calculated as mean ± SEM. Significance was established with the SPSS statistical (SPSS, USA) and GraphPad Prism 5 software (GraphPad Software, USA), Pearson's correlation coefficient or a two-tailed Student's t-test. The data are presented as the mean and SEM of three independent experiments. Differences were considered significant at *P<0.05, **P<0.01.
Results
GON4L expression and effects of GON4L overexpression in NPC cells growth and proliferation
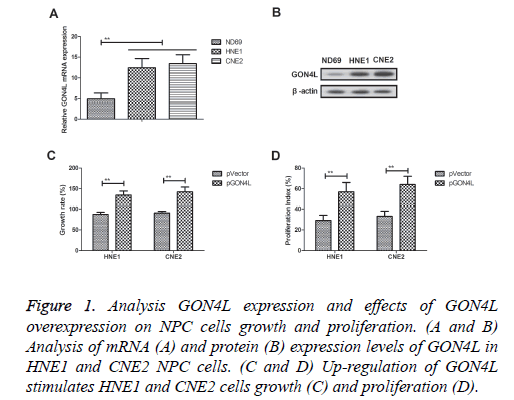
GON4L mRNA and protein expression levels were detected in HNE1 and CNE2 NPC cells (Figures 1A and 1B). Up-regulation of GON4L mRNA and protein expression levels was observed in both HNE1 and CNE2 cells compared to human nasopharyngeal epithelial cells (ND69). Results demonstrated that GON4L overexpression (pGON4L) stimulated HNE1 and CNE2 cells growth and proliferation (Figures 1C and 1D). These results indicate GON4L is overexpressed in NPC cells and its overexpression promotes growth and proliferation of NPC cells.
Figure 1: Analysis GON4L expression and effects of GON4L overexpression on NPC cells growth and proliferation. (A and B) Analysis of mRNA (A) and protein (B) expression levels of GON4L in HNE1 and CNE2 NPC cells. (C and D) Up-regulation of GON4L stimulates HNE1 and CNE2 cells growth (C) and proliferation (D).
Knockdown of GON4L expression impairs NPC cells growth and proliferation
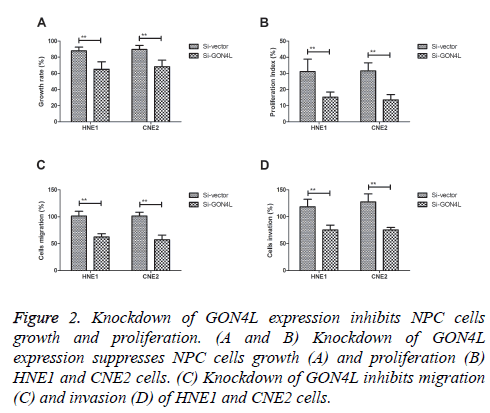
In order to analyse effects of GON4L on the progression of NPC cells, colony formation and proliferation was investigated in GON4L-knockdown (Si-GON4L) NPC cells. As shown in Figures 2A and 2B, Knockdown of GON4L expression suppressed NPC cells growth and proliferation. Results demonstrated that knockdown of GON4L inhibited migration and invasion of HNE1 and CNE2 cells (Figures 2C and 2D). These results suggest that down-regulation of GON4L expression can impair NPC cells growth, proliferation and aggressiveness.
Knockdown of GON4L expression enhances cisplatin-induced apoptosis of NPC cells
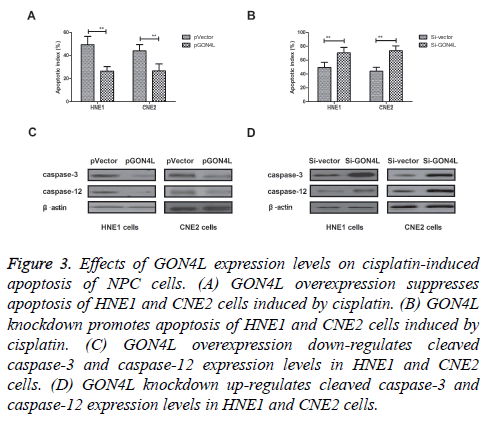
Effects of GON4L expression on apoptosis of NPC cells induced by cisplatin were investigated in this work. We found that GON4L overexpression inhibited apoptosis of HNE1 and CNE2 cells induced by Cisplatin (Figure 3A). However, knockdown of GON4L expression by Si-GON4L promoted Cisplatin-induced apoptosis of NPC cells compared to control (Figure 3B). Western blot showed that cleaved caspase-3 and caspase-12 were down-regulated by GON4L overexpression, while up-regulated by GON4L knockdown (Figures 3C and 3D). These results present GON4L expression can negatively regulated Cisplatin-induced apoptosis of NPC cells.
Figure 3: Effects of GON4L expression levels on cisplatin-induced apoptosis of NPC cells. (A) GON4L overexpression suppresses apoptosis of HNE1 and CNE2 cells induced by cisplatin. (B) GON4L knockdown promotes apoptosis of HNE1 and CNE2 cells induced by cisplatin. (C) GON4L overexpression down-regulates cleaved caspase-3 and caspase-12 expression levels in HNE1 and CNE2 cells. (D) GON4L knockdown up-regulates cleaved caspase-3 and caspase-12 expression levels in HNE1 and CNE2 cells.
Knockdown of GON4L expression inhibits growth and proliferation of NPC cells through β-catenin/wnt signaling pathway
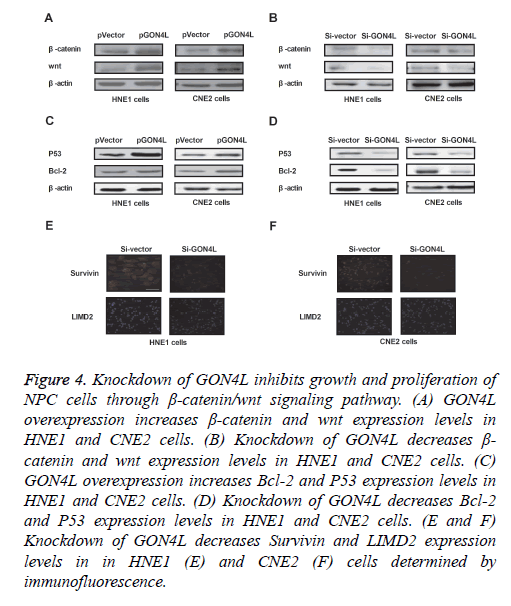
We further analysed GON4L-mediated potential mechanism in NPC cells. As shown in Figures 4A and 4B, expression levels of β-catenin and wnt were up-regulated by GON4L overexpression and down-regulated by GON4L knockdown in HNE1 and CNE2 cells. GON4L knockdown also suppressed Bcl-2 and P53 expression levels in HNE1 and CNE2 cells (Figures 4C and 4D). Immunofluorescence demonstrated that Survivin and LIMD2 were decreased by Si-GON4L in HNE1 and CNE2 cells (Figures 4E and 4F). These results indicate knockdown of GON4L inhibits growth and proliferation of NPC cells through down-regulation of β-catenin/wnt signaling pathway.
Figure 4: Knockdown of GON4L inhibits growth and proliferation of NPC cells through β-catenin/wnt signaling pathway. (A) GON4L overexpression increases β-catenin and wnt expression levels in HNE1 and CNE2 cells. (B) Knockdown of GON4L decreases β- catenin and wnt expression levels in HNE1 and CNE2 cells. (C) GON4L overexpression increases Bcl-2 and P53 expression levels in HNE1 and CNE2 cells. (D) Knockdown of GON4L decreases Bcl-2 and P53 expression levels in HNE1 and CNE2 cells. (E and F) Knockdown of GON4L decreases Survivin and LIMD2 expression levels in in HNE1 (E) and CNE2 (F) cells determined by immunofluorescence.
Discussion
NPC is a rare cancer with regional allocations, but presents relative higher metastasis among human tumors [19,20]. GON4L has been identified as transcriptional repressor that can be as a molecular target for bladder cancer therapy [12]. In addition, inhibition of β-catenin/wnt singling pathway contributes to suppress growth and proliferation of nasopharyngeal carcinoma [21,22]. In this study, we assumed that GON4L regulates growth and proliferation through β- catenin/wnt singling pathway in nasopharyngeal carcinoma cells. Findings have suggested that GON4L is overexpressed in NPC cells and overexpression of GON4L promotes growth and proliferation as well as inhibits apoptosis of NPC cells induced by Cisplatin. Notably, knockdown of GON4L significantly inhibits growth and proliferation, which further enhances apoptosis of NPC cells through up-regulation of cleaved caspase-3 and caspase-12 and down-regulation of P53 and Bcl-2 expression levels.
Growth and proliferation of NPC cells is associated with metastatic characteristic and apoptosis resistance via alteration of cell cycle progression [23]. Previous reports have indicated that Survivin is overexpressed in nasopharyngeal carcinoma, resulting in enhancement of apoptosis resistance and rapid proliferation of NPC cells [24,25]. Cai et al. have showed that Survivin gene functions is related with prognosis in patients with nasopharyngeal carcinoma, which may be helpful for prognostic and anti-cancer treatment appraisal [26]. Peng et al. have indicated that LIMD2 is a novel small LIM-only protein related tumor migration and invasion, which is overexpressed in metastatic lesions and can promote tumor cells aggressiveness by directly binding to and activating the integrin-linked kinase [27]. In this study, results revealed that knockdown of GON4L significantly inhibits expression levels of Survivin and LIMD2 in HNE1 and CNE2 cells, which may contribute to inhibition of growth and proliferation. These investigations suggest that GON4L is associated with NPC cells growth and proliferation by inhibition of Survivin and LIMD2 expression levels in vitro.
Currently, β-catenin/wnt signal pathway is associated with growth and proliferation of NPC cells that up-regulation of β- catenin and wnt expression enhances the resistance of tumor cells apoptosis [28,29]. Report has indicated that β-catenin is important for cancer stem cell generation and tumorigenic activity in nasopharyngeal carcinoma [30]. Yu et al. also indicated that genetic polymorphisms of Wnt/beta-catenin pathway genes are associated with the efficacy and toxicities of radiotherapy in patients with nasopharyngeal carcinoma, which may be novel prognostic factors for NPC patients treated with radiotherapy [31]. In this study, we have found that GON4L regulates growth and proliferation by down-regulation of β- catenin/wnt signal pathway in NPC cells. GON4L knockdown significantly inhibits β-catenin and wnt expression levels, which further enhances Cisplatin-induced apoptosis of NPC cells.
In conclusion, this study we found that GON4L can regulate the colony formation and proliferation of NPC, which can be regarded as an anti-tumor target gene. Findings have indicated that GON4L expression level is up-regulated in NPC cells. However, knockdown of GON4L can significantly inhibit the proliferation and migration of HNE1 and CNE2 cells. These results indicate that GON4L is associated with NPC proliferation and aggressiveness, so it may provide a strategy for exploring molecular mechanism and tumor targeting therapy for NPC patients.
References
- Petersson F. Nasopharyngeal carcinoma: a review. Semin Diagn Pathol 2015; 32: 54-73.
- Co J, Mejia MB, Dizon JM. Evidence on effectiveness of intensity-modulated radiotherapy versus 2-dimensional radiotherapy in the treatment of nasopharyngeal carcinoma: Meta-analysis and a systematic review of the literature. Head Neck 2016; 38: E2130-2142.
- Zhang LF, Li YH, Xie SH. Incidence trend of nasopharyngeal carcinoma from 1987 to 2011 in Sihui County, Guangdong Province, South China: an age-period-cohort analysis. Chin J Cancer 2015; 34: 350-357.
- Liu S, Wang X, Shu J, Zhao Z, Sun Z, Luo B. Sequence analysis of EBV immune evasion gene BNLF2a in EBV associated tumors and healthy individuals from nasopharyngeal carcinoma endemic and non-endemic regions of China. J Med Virol 2015; 87: 1946-1952.
- Tian W, Zhu FM, Wang WY. Sequence-based typing of HLA-A gene in 930 patients with nasopharyngeal carcinoma in Hunan province, southern China. Tissue Antigens 2015; 86: 15-20.
- Tsang CM, Tsao SW. The role of Epstein-Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. Virol Sin 2015; 30: 107-121.
- Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of Nasopharyngeal Carcinoma (NPC). Sem Cancer Biol 2012; 22: 144-153.
- Turki S, Mardassi A, Abouda M, Hachicha A, Ben Jallel W. Orbital metastasis revealing an undifferentiated Carcinoma of nasopharyngeal type: a case report. Tunis Med 2016; 94: 148-151.
- Genova P, Brunetti F, Bequignon E. Solitary splenic metastasis from nasopharyngeal carcinoma: a case report and systematic review of the literature. World J Surg Oncol 2016; 14: 184.
- Liu TH, Zheng F, Cai MY. The putative tumor activator ARHGEF3 promotes nasopharyngeal carcinoma cell pathogenesis by inhibiting cellular apoptosis. Oncotarget 2016; 7: 25836-25848.
- Lu P, Hankel IL, Hostager BS. The developmental regulator protein Gon4l associates with protein YY1, co-repressor Sin3a, and histone deacetylase 1 and mediates transcriptional repression. J Biol Chem 2011; 286: 18311-18319.
- Agarwal N, Dancik GM, Goodspeed A, Costello JC, Owens C. GON4L drives cancer growth through a YY1-androgen receptor-CD24 axis. Cancer Res 2016; 76: 5175-5185.
- Lu P, Hankel IL, Knisz J, Marquardt A, Chiang MY. The Justy mutation identifies Gon4-like as a gene that is essential for B lymphopoiesis. J Exp Med 2010; 207: 1359-1367.
- Lowy AM, Clements WM, Bishop J. Beta-Catenin/Wnt signaling regulates expression of the membrane type 3 matrix metalloproteinase in gastric cancer. Cancer Res 2006; 66: 4734-4741.
- Suksaweang S, Lin CM, Jiang TX, Hughes MW, Widelitz RB, Chuong CM. Morphogenesis of chicken liver: identification of localized growth zones and the role of beta-catenin/Wnt in size regulation. Develop Biol 2004; 266: 109-122.
- Weinberger PM, Adam BL, Gourin CG. Association of nuclear, cytoplasmic expression of galectin-3 with beta-catenin/Wnt-pathway activation in thyroid carcinoma. Arch Otolaryngol Head Neck Surg 2007; 133: 503-510.
- Lacroix-Triki M, Geyer FC, Lambros MB. Beta-catenin/Wnt signalling pathway in fibromatosis, metaplastic carcinomas and phyllodes tumours of the breast. Modern Pathol Off J US Canad Acad Pathol 2010; 23: 1438-1448.
- Renshaw A, Elsheikh TM. A validation study of the Focalpoint GS imaging system for gynecologic cytology screening. Cancer Cytopathol 2013; 121: 737-738.
- Tao CJ, Chen YY, Jiang F. A prognostic model combining CD4/CD8 ratio and N stage predicts the risk of distant metastasis for patients with nasopharyngeal carcinoma treated by intensity modulated radiotherapy. Oncotarget 2016; 7: 46653-46661.
- Wang JY, Li XF, Li PZ, Zhang X, Xu Y, Jin X. MicroRNA-23b regulates nasopharyngeal carcinoma cell proliferation and metastasis by targeting E-cadherin. Mol Med Reports 2016; 14: 537-543.
- Wang FL, Guo X, Yuan TZ, Cao SM, Rao HL. Expression and clinical significance of Wnt-1 and beta-catenin in nasopharyngeal carcinoma. Ai Zheng 2009; 28: 72-75.
- Galera-Ruiz H, Rios-Moreno MJ, Gonzalez-Campora R, Galera-Davidson H. WNT pathway in laryngeal squamous cell carcinoma and nasopharyngeal carcinoma. Acta Otorhinolaryngologica ITALICA Organo Ufficiale Della SOCIETA Italiana Di Otorinolaringologia E Chirurgia Cervico-Facciale 2012; 32: 122-123.
- Yeh KY, Chang JW, Li YY, Wang CH, Wang HM. Tumor growth inhibition of metastatic nasopharyngeal carcinoma cell lines by low dose of arsenic trioxide via alteration of cell cycle progression and induction of apoptosis. Head Neck 2011; 33: 734-742.
- Li YH, Hu CF, Shao Q. Elevated expressions of survivin and VEGF protein are strong independent predictors of survival in advanced nasopharyngeal carcinoma. J Transl Med 2008; 6: 1.
- Faqing T, Zhi H, Liqun Y. Epstein-Barr virus LMP1 initiates cell proliferation and apoptosis inhibition via regulating expression of Survivin in nasopharyngeal carcinoma. Exp OncoL 2005; 27: 96-101.
- Cai JH, Fu SM, Tu ZH. Survivin gene functions and relationships between expression and prognosis in patients with nasopharyngeal carcinoma. Asian Pac J Cancer Prevent APJCP 2015; 16: 2341-2345.
- Peng H, Talebzadeh-Farrooji M, Osborne MJ. LIMD2 is a small LIM-only protein overexpressed in metastatic lesions that regulates cell motility and tumor progression by directly binding to and activating the integrin-linked kinase. Cancer Res 2014; 74: 1390-1403.
- Zhang J, Wen X, Ren XY. YPEL3 suppresses epithelial-mesenchymal transition and metastasis of nasopharyngeal carcinoma cells through the Wnt/beta-catenin signaling pathway. J Exp Clin Cancer Res CR 2016; 35: 109.
- Song Y, Yang QX, Zhang F, Meng F, Li H. Suppression of nasopharyngeal carcinoma cell by targeting β-catenin signaling pathway. Cancer Epidemiol 2012; 36: e116-121.
- Jiang R, Niu X, Huang Y, Wang X. Beta-Catenin is important for cancer stem cell generation and tumorigenic activity in nasopharyngeal carcinoma. Acta Biochimica Et Biophysica Sinica 2016; 48: 229-237.
- Yu J, Huang Y, Liu L. Genetic polymorphisms of Wnt/beta-catenin pathway genes are associated with the efficacy and toxicities of radiotherapy in patients with nasopharyngeal carcinoma. Oncotarget 2016.