ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 2
Green synthesis, characterization and anxiolytic, sedative and hypnotic activity of pyrimidine based diazepine derivatives
1Department of Chemistry, Manonmaniam Sundaranar University, Tirunelveli, Tamilnadu, India
2Department of Chemistry, Sri Vidhya Mandir College, Uthangarai, Tamilnadu, India
3Department of Chemistry, Islamiah College, Vaniyambadi, Tamilnadu, India
4Department of Chemistry, Adhiyamaan College of Engineering, Hosur, Tamilnadu, India
- *Corresponding Author:
- Govindaraj Chinnasamy
Department of Chemistry
Manonmaniam Sundaranar University, India
Accepted on May 10, 2016
Synthesis of Pyrimidino-4,6-(2,4-Diazepine) derivatives were achieved by green and one pot synthesis using 1,2-diketones and 4,6-diamino Pyrimidines as the precursor of condensation reaction and CTAB as the cationic surfactant. For the purpose of determining the efficiency of substituent on 1,2-diketones, three different forms of diketones were used viz., Benzil, 4,4'-dimethyl Benzil and 4,4'-difluoro Benzil. The results showed that the use of 4,4'-difluoro Benzil, was better among the three Benzils, considering its yield and the time consumption. All the three types of Benzodiazepine derivatives so obtained were independently used for evaluating their anxiolytic, sedative and hypnotic activity using albino mice as the subject. When the resultant Pyrimidino-benzodiazepines was compared with standard Diazepam, the following results were obtained: Sedative and Hypnotic activity >Standard Diazepam, but Anxiolytic activity< Standard Diazepam. In most cases, Fluoro substituted Diazepine show deviation in activity. The results in locomotor activity suggest the appreciable alertness of the mice when exposed to Pyrimidine based Diazepine drugs.
Keywords
CTAB, Condensation reaction, Pyrimidino diazepines, Biological activity.
Introduction
Benzodiazepines have been largely used for the past few decades to treat different forms of CNS disorders, but due to their unwanted side effects, alternative treatment strategies with favorable side-effect profiles, credible benefits and moderate costs are of interest, especially in primary care settings. Synthetic compounds are a good source to find new remedies for these disorders. In the search for an alternative, more specific, and perhaps cost-free therapy, research is being intensively conducted to investigate the anxiolytic, sedative and hypnotic and locomotor activity of the synthesized compounds. Benzodiazepines have anti-carcinogenic [1] and analgesic effect [2], 2-amino Pyrimidines are anti-histaminic [3] and few Pyrimidine derivatives are known to exhibit antiangiogenic and anti-tumor activity [4]. Moreover, N-hetero compounds and their derived compounds are of fundamental importance for the preparation of most efficient ligands and organocatalysts [5]. Moreover, Pyrimidine based diazepine is more potent anxiolytic than dibenz-(1,4)-diazepin-1-one analogues [6]. The structure of the Benzodiazepine molecules especially, the seven membered ring systems, present in their structure, play an important role in the biological activity of these molecules [7]. These systems are known for their specificity in action. The core aim of this research work is to prepare Benzodiazepine derivatives with 4, 6-diamino Pyrimidine as one of the precursors, using a quick and green way and to evaluate their applications as Anxiolytic, Sedative and Hypnotic drug using Albino mice as the subject. Usually, benzodiazepines are prepared by the condensation of 4, 6- diamino Pyrimidines using 1, 2-diketone in refluxing ethanol for 2-12 hours or by some other methods like solid phase synthesis, which gives only 34-70% yields [8-10]. We have achieved quick synthesis of Pyrimidino-benzodiazepine by using Benzil, 4,4’-dimethylBenzil and 4,4’-difluroBenzil as precursors in the presence of surfactants at 35°C. The major green synthesis requirement also falls on the use of appropriate solvent. The usage of water as the reaction medium ensures this requirement [11].
Materials and Methods
Chemistry
Melting points were determined using a capillary in a melting point apparatus (Toshiba) and are uncorrected. The IR Spectra were recorded on a Perkin Elmer Spectrum1 FT-IR instrument with a typical resolution of 1.0 cm-1 using the KBr disc in the range of 4000-400 cm-1. The H1 and 13C NMR spectra were measured on a Bruker Advance III instrument (600 and 150 MHz, resp.) using a solvent signal as the reference. Chemical shifts (δ) are given in ppm and coupling constants (J) in Hz. Assignments of signals in 13C NMR spectra were made on the basis of HMQC experiments.
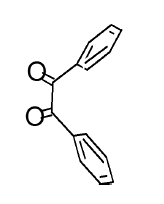
Both Pyrimidine (99.9%), 1,2-diketones (99.8%) were obtained from Sigma-Aldrich, India and were used as such without further purification. 1,2-diketone was used in three different forms viz., Benzil, 4,4’-dimethylBenzil and 4,4’-difluroBenzil for the purpose of finding the substituent effect. All these di-ketones were intentionally used in the form of 1,2-aryl di-ketones rather than any form of alkyl diketones, because of their unsuitability for the preparation of Diazepines. CTAB was used as the surfactant. A mixture of 1 M HCl and Ethanol-spirit was used as the solvent. After completion of the reaction (which was completely monitored by TLC), the synthesized product was precipitated by using spirit-ethanol. Surfactant (CTAB) was left in the solvent.
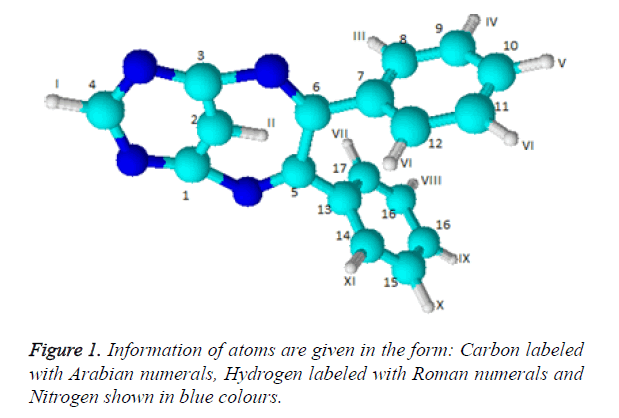
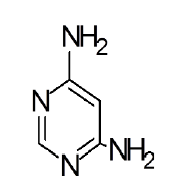
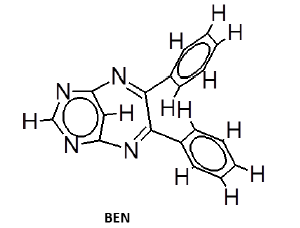
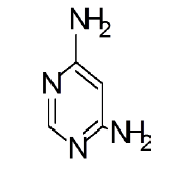
General procedure for synthesis of pyrimidino-4,6-(2,4- Diazepine) derivatives: In a 100 ml beaker, 2 mmol of Benzil, 2 mmol of CTAB were added to 10 ml Ethanol and placed on a magnetic stirrer cum temperature bath at stirring speed of 750 rpm at 35°C. 10 ml of 4,6-diaminoPyrimidine in 1 M HCl was added in-situ to this reaction mixture drop by drop. A yellow coloured precipitate was obtained after 10 minutes. The precipitate was filtered using Whatmann 40 filter paper and dried under shadow. The precipitate was re-crystallized with Spirit-Ethanol. This re-crystallized yield was used for further characterization. The structure of the resulting compound was as shown in the Figure 1.
Procedure for the synthesis of 3-phenyl-2,5,7,9-tetraazabicyclo[ 4.3.1]deca-1(9),2,4,6(10),7-pentaene; compound with toluene: In a 100 ml beaker, 2 mmol of 1,2-diphenyl-ethane- 1,2-dione, 2 mmol of CTAB were added to 10 ml Ethanol and placed on magnetic stirrer, added with 10 ml of 4,6-diaminoPyrimidine in 1 M HCl, in-situ to this reaction mixture, drop by drop. The precipitate was re-crystallized with Spirit-Ethanol.
Characterization of 3-phenyl-2,5,7,9-tetraaza-bicyclo[ 4.3.1]deca-1(9),2,4,6(10),7-pentaene; compound with toluene: Yield=85%: Yellow coloured crystals: M.P=99°C; IR :-C=N str.=1583.58 cm-1, aromatic -CHstr= 3064.89 cm-1, ; H1NMR : (400 MHz, DMSO): δ=7.927-7.946 (J=1.979) (d, 4H, arom (C-8,12,14,18)); δ =7.790-7.827 (J=1.90) (t, 2H, arom (C-10,16); δ = 7.622-7.659 (J=1.975) (t, 4H, arom (C-9,11,15,17)); δ =3.444 (s, 1H, arom (C-2); δ =3.122(s, 1H, arom (C-4); C13NMR: (400 MHz, DMSO) δ = 194.8, 135.53, 132.21, 129.57, 129.49, 40.13, 39.92, 39.71, 39.5, 39.29, 39.09, 38.88.
Procedure for the synthesis of 3-p-Tolyl-2,5,7,9-tetraaza-bicyclo[ 4.3.1]deca-1(9),2,4,6(10),7-pentaene; compound with p-xylene: In a 100 ml beaker, 2 mmol of 1,2-Di-p-tolyl-ethane- 1,2-dione, 2 mmol of CTAB were added to 10 ml Ethanol and placed on magnetic stirrer, added with 10 ml of 4,6-diaminoPyrimidine in 1 M HCl, in-situ to this reaction mixture, drop by drop. The precipitate was re-crystallized with Spirit-Ethanol.
Characterization of 3-p-Tolyl-2,5,7,9-tetraaza-bicyclo[ 4.3.1]deca-1(9),2,4,6(10),7-pentaene; compound with p-xylene: Yield = 87%: Yellow coloured crystals: M.P.=107°C; IR :-C=N str. =1598.99 cm-1, aromatic -CH-str.= 3055.24 cm-1; H1NMR:(400 MHz, DMSO) δ = 7.790-7.807 (J=1.97) (d, 4H, arom, C-8,12, 14, 18); δ =7.408-7.425(J=2.00) (d, 4H, arom, C-9,11,15,17); δ = 3.409 (s, 1H, arom, C-2); δ =2.402 (s, 1H, arom, C-4); C13NMR: (400 MHz, DMSO) δ = 194.61, 146.45, 130.02, 129.91, 129.72, 129.6, 40.12, 39.91, 39.7, 39.5, 39.29, 39.08, 39.87, 21.41
Procedure for the synthesis of 3-(4-Fluoro-phenyl)-2,5,7,9- tetraaza-bicyclo[4.3.1]deca-1(9),2,4,6(10),7-pentaene; compound with 1-fluro-4-methyl-benzene: In a 100 ml beaker, 2 mmol of 1,2-Bis-(4-flouro-phenyl)-ethane-1,2-dione, 2 mmol of CTAB were added to 10 ml Ethanol and placed on magnetic stirrer, added with 10 ml of 4,6-diaminoPyrimidine in 1 M HCl, in-situ to this reaction mixture, drop by drop. The precipitate was re-crystallized with Spirit-Ethanol.
Characterization of 3-(4-Fluoro-phenyl)-2,5,7,9-tetraaza-bicyclo[ 4.3.1]deca- 1(9),2,4,6(10),7-pentaene; compound with 1-fluro-4-methyl-benzene: Yield=86%: Yellow coloured crystals. M.P.=118°C; IR:-C=N str. =1595.13cm-1, aromatic - CH str. = 3084.18 cm-1; H1NMR (400 MHz, DMSO) δ = 8.031-8.065 (J=1.00) (dt, 4H, arom, C-9,11, 15,17); δ = 7.447-7.490 (J=0.994) (t, 4H, arom, C-8,12, 15, 17); δ =3.384 (s, 1H, arom, C-2); δ =3.131 (s, 1H, arom, C-4).; C13NMR (400 MHz, DMSO) δ = 192.59, 167.51, 164.97, 133.05, 132.95, 128.99, 128.97, 116.86, 116.63, 40.12, 39.91, 39.70, 39.49, 39.28, 39.08, 38.87.
Pharmacology
Animals: Male Swiss albino mice (20-25 g) were used throughout the study. They were provided normal pellet diet and tap water ad libitum and were exposed to 12 h light and 12 h dark cycle. The animals were acclimatized to the laboratory conditions before experiments. Care of the animals was taken as per guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India.
Drugs: Diazepam hydrochloride (Calmpose injection, Ranbaxy Laboratories, Gurgaon, India) was procured and used as a standard drug and it was diluted with saline to the required strength before use. The test compounds were suspended in 0.3% w/v of sodium carboxymethyl cellulose (CMC) and were administered through oral gavage.
Acute toxicity studies: Acute Oral Toxicity (AOT 423) guideline was followed for the study of acute toxicity of the synthesized compounds [12]. Briefly, overnight fasted animals were treated with various concentrations of the test compounds (5, 50, 300 and 2000 mg/kg, bw) by oral route. The animals were observed for any behavioral changes or mortality for first 24 h. The animals were maintained for another 14 days to check the abnormal changes and mortality. Based on the results, the dose was fixed for pharmacological studies.
Anxiolytic activity-elevated plus maze (EPM) test: To assess the anti-anxiety activity of test compounds, elevated plus maze (EPM) was used [13]. The plus-maze apparatus consisting of two open arms (16 × 5 cm) and two closed arms (16 × 5 × 12 cm) having an open roof, with the plus-maze elevated (25 cm) from the floor. Each mouse was placed at the centre of the elevated plus maze with its head facing the open arms. During this 5 minutes experiment, the behavior of the mouse was recorded as: (a) the number of entries into the open arms, (b) average time spent by the mouse in the open arms (average time=total time spent in open arms/number of entries in arms).
Procedure: Animals were divided in to 11 groups, each 6 in a group. The Group I served as control served with vehicle, Group II served as standard served with diazepam at the dose of 0.5 mg/kg. Group III-XI the animals were treated with the test compounds at the dose of (5 mg/kg, bw, p.o). The compounds were administered orally using a tuberculin syringe fitted with oral cannula. The dose administration schedule was so adjusted that each mouse was having its turn on the elevated plus-maze apparatus 45 minutes after the administration of the dose. During the entire experiment, the animals were allowed to socialize. Every precaution was taken to ensure that no external stimuli, other than the height of plus-maze could invoke anxiety in the animals. The parameters observed is (a). Time spent in open arm and (b). Number of arm entries in order to assess the anxiolytic activity.
Sedative and Hypnotic activity-Hole - Board Test: To evaluate the sedative and hypnotic activity of the synthesized compounds, Hole-Board Test was performed [14,15]. The hole-board apparatus comprised a grey Perspex panel (40 × 40 cm, 2.2 cm thick) with 16 equidistant holes (3 cm diameter) in the floor. Photocells below the surface of the holes provided the measure of the number of head dips. The board was positioned 15 cm above the table and was divided with black water-resistant marker into 9 squares of 10 × 10 cm. Thirty minutes after the administration of the test drug, each mouse was individually placed in the centre of the board (facing away from the observer). During 5 min test period, number of head dips was noted.
Procedure: Animals were divided in to 11 groups, each 6 in a group. The Group I served as control served with vehicle, Group II served as standard served with diazepam at the dose of 3 mg/kg. Group III-XI the animals were treated with the test compounds at the dose of (5 mg/kg, bw, p.o). The test compounds were administered orally using a tuberculin syringe fitted with oral canola. After 45 minutes of oral administration of the test compounds, the animals were placed in to the center of the perforated board and observed for the period of 5 minutes and the number of head exploration was counted in order to find out the sedative and hypnotic activity.
Locomotor Activity: To evaluate the spontaneous locomotor activity of the synthesized compounds, actophotometer was used [16]. Actophotometer (24 × 22 × 10 cm) (Popular Traders, Ambala) with automatic counting of animal movements in the floor of activity cage was utilized for the present study. Mice were placed individually in activity cage for 5 min test period, 45 min after administration of test drugs. Locomotor activity was observed in terms of the activity scores.
Procedure: Animals were divided in to 11 groups, each group consists of 6 animals. The Group I served as control served with vehicle, Group II animals served as standard which receives diazepam at the dose of 3 mg/kg, through intra-peritoneal route. Group III-XI the animals were treated with the test compounds at the dose of (5 mg/kg, bw, p.o). The test compounds were administered orally using a tuberculin syringe fitted with oral cannula. After 45 minutes of oral administration of the various fractions the animals were placed in to the actophotometer. Movement of the animal is recorded by the light beams present inside the actophotometer for the evaluation of the locomotor activity.
Results and Discussion
Synthesis and characterizations
Condensation of 4, 6-diamino Pyrimidine with Benzil was employed as the key reaction. Four kinds of polar mixtures as mentioned in the Table 1 were used as the solvent and tested independently for their efficiency. Acrylonitrile resulted in better yield and consumed least time (<4 minutes). Water as the solvent resulted in poor yield and the more time consuming among its counterparts used. Although Acrylonitrile was useful, ethanol was chosen as the solvent for all the rest of synthesis. This was owing to the less toxic, more available, economical and efficiency reasons of ethanol over the others.
| S.No. | Solvent | CTAB (mmol) | % Yield | Time in minutes |
|---|---|---|---|---|
| 1. | Ethanol/HCl | 2 | >95% | 25 min |
| 2. | Methanol/HCl | 2 | >90% | 30 min |
| 3. | H2O/HCl | 2 | 80% | 40 min |
| 4. | CH3CN | 2 | 97% | <4 min. |
Table 1. Effect of solvents.
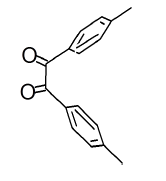
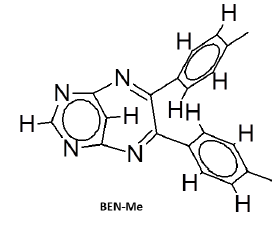
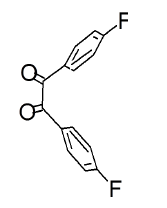
The mesomeric effect of para substituent present on Benzil was verified by varying different types of groups and the corresponding results are listed in Table 2. Electron withdrawing substituent such as -Fluro, yielded appreciably (Scheme III), while the electron donating groups such as Methyl, (Schemes II) slightly affected the yield and time consuming as well. The impact of applying cationic surfactant on the reaction was determined by using CTAB, owing to its advantage in synthesis [17]. All the reaction schemes I, II and III yielded better and required short timing since, CTAB was used and their corresponding structures were confirmed by spectroscopic data.
| Pyrimidine | Benzil | Benzodiazepine | Yield | #Time | Scheme |
|---|---|---|---|---|---|
 |
 |
 |
96% | a.5 min b.35min |
(I) |
 |
 |
 |
93% | a.7 min b.45min |
(II) |
 |
 |
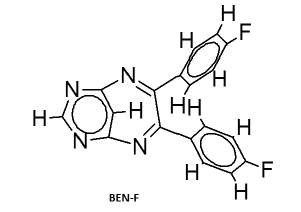 |
97% | a.3 min b.30min |
(III) |
| #NOTE: In the column Time, ‘a’ refers to reaction carried out in the presence of CTAB; ‘b’ refers to the same reaction carried out in the absence of CTAB. | |||||
Table 2. Reaction schemes.
Acute toxicity studies
Acute oral toxicity study for the test compounds were determined by using AOT 423 guidelines. Two out of three animals were died in the group of animals treated with 300 mg/kg, bw, hence the preceding dose was considered as LD50 dose. One tenth of the dose i.e. 5 mg/kg, bw was used for further studies.
Anxiolytic activity
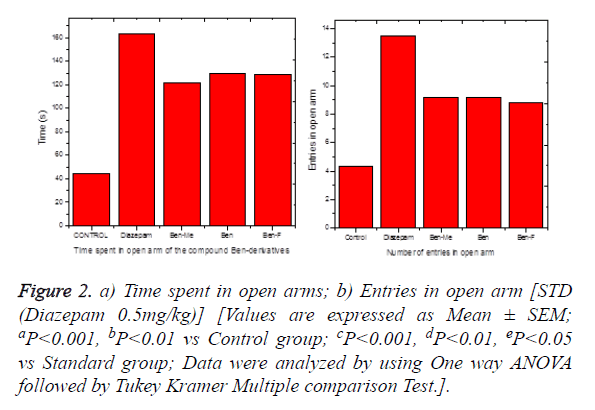
The time spent in the open arms by the animals treated with standard drug diazepam and test compounds was significantly increased when compared to the control group which was treated with the same volume of vehicle. Among the test compounds, all the three substituted benzodiazepine series showed significant activity, which shows mild to moderate activity. These compounds do not show any significant difference when compared to the standard drug which indicates that the compounds are equipotent to the standard drug used i.e., diazepam. The results are shown in the Figures 2a and 2b. The time spend in the open arm is greatly increased and the number arm entry also gradually increased based on the potency of the test compounds. Since, increases in open arm entry parameters are the most representative indices of anxiolytic activity. Time spent on the central platform appears to be related to decision making or risk assessment, and the total arm entries is a measure reflecting changes in anxiety or in general activity. The interconvulsant, anxiolytic and sedative effects of benzodiazepines and their derivatives are good candidates for enhancing the action of Gama Amino Butyric Acid (GABAA) [18]. Benzodiazepines bind to the gamma subunit of GABAA receptor, due to which a desired structural modification of the receptor results, which leads to an increase in GABAA receptor activity [19].
Figure 2. a) Time spent in open arms; b) Entries in open arm [STD (Diazepam 0.5mg/kg)] [Values are expressed as Mean ± SEM; aP<0.001, bP<0.01 vs Control group; cP<0.001, dP<0.01, eP<0.05 vs Standard group; Data were analyzed by using One way ANOVA followed by Tukey Kramer Multiple comparison Test.].
This activity might be due to the action of the test compounds directly on the brain GABAnergic system. These drug probably binding to the benzodiazepine binding site of the ion channel receptor present in the CNS, which in turn stimulate the binding of GABAA subunit with the GABA receptor, which in turn produce the prolongation of opening of the chloride ion channel leads to mild hyper polarization which might be responsible for the anti-anxiety activity of these test compounds. Benzodiazepines act not by substituting for GABAA, which bind at the α-subunit, but increase the frequency of channel opening events, which leads to an increase in Chloride ion conductance and also inhibition of the action potential [20]. There are believes that the anxiolytic action of benzodiazepine may be due to the direct activation of glucone synapses in the brain [21].
Sedative and hypnotic activity
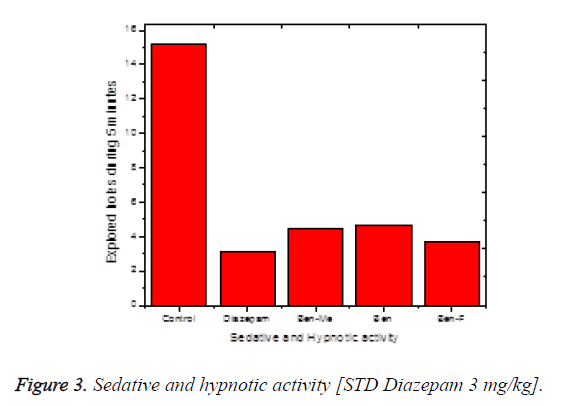
The hole-board test used to assess the sedative and hypnotic potential of the test compounds. This test confirmed the CNS calming nature of the test compounds. The results are shown in the Figure 3. The number of hole explore was counted for the period of 5 minutes and the results revealed that the control animal was active whereas the head exploration has been largely reduced in the animals treated with the standard diazepam drug at the dose of 4 mg/kg. The test compounds possess various degree of hole exploration values. Diazepam is CNS depressant used in the management of sleep disorders such as insomnia [21]; these compounds have a binding site on GABA receptor type-A ionophore complex (GABAA). It decreases activity, moderates excitement and calms the recipient. Since GABAergic transmission can produce profound sedation in mice. The inhibitory action of GABA consists in the opening of chloride channels to allow hyperpolarizing the membrane, leading to CNS depression and resulting in sedative and hypnotic activity. Glutamate and GABA are quantitatively the most important excitatory and inhibitory neurotransmitters, respectively, in the mammalian brain [21]. Thus, receptors for these two neurotransmitters are regarded as important targets for psychotropic drugs. The activity of the test compound may be due to binding with these receptors.
There is evidence for the derivatives of annulated Pyrrolo (1,4) benzodiazepines to exhibit sleep inducing activity and anxiolytic activity almost equivalent to that of standard Diazepam [22]. But our test compounds exhibits better sedative property than standard Diazepines. The order of sedative activity was, BEN-Me>BEN>BEN-F>standard Diazepam (Figure 3). The reason for BEN-Me exhibiting the superior activity could be, the presence of +mesomeric functional groups. In this Sedative activity aspect, tetracyclic benzodiazepines like Bretazenil are inferior, when compared with our tricyclic Pyrimidino-benzodiazepines [23].
Locomotor activity
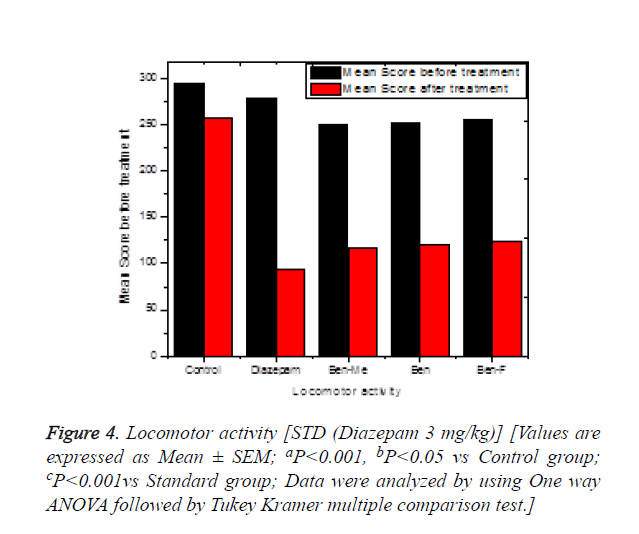
In the locomotor activity, the mean score before and after treatment were measured and are shown in the Figure 4. There is significant difference observed in the mean score before and after treatment with standard and test compounds. After the standard/test drug treatment, the mean score for the diazepam treated group and test compounds treated, shows significant activity when compared to control group and slightly better statistical significance was observed when compared to the standard diazepam. There was very slight variation among the test compounds and standard and the order of mean score after the treatment was found to be BEN-F>BEN>BEN-Me> Diazepam. The results observed revealed that the compounds significantly reduce the locomotor activity. Locomotor activity is considered as an index of alertness and a reduction is indicative of sedative activity. GABA is the major inhibitory neurotransmitter in CNS and different anxiolytic, muscle relaxant and sedative-hypnotic drugs exhibit their action via GABA [24]. Therefore, it is possible that test compounds may act by potentiating GABAergic inhibition in the CNS via membrane hyper-polarization leading to a reduction in the firing rate of critical neurons in the brain or it may be due to direct activation of GABA receptors by the test compounds. The increase in efficiency of the natural brain chemical, GABAA, by the Benzodiazepines is achieved along with the decrease in excitability of neurons [24].
Conclusion
Pyrimidino-Diazepines were successfully synthesized with greater yield and shorter duration using CTAB as the surfactant. The synthesis in the absence of cationic surfactant resulted in poor yield and time consuming. The solubility of this compound in ethanol, suggests that this product can be used as a challenging drug for the pathogens. Moreover, the synthesis of Pyrimidino-Diazepine by this method produced no harmful emissions. These provide a very good option for non-toxic drug possessing better anxiolytic, sedative and hypnotic properties than a few of the reported tricyclic compounds. When compared with Diazepam, these Pyrimidine based drugs shows a little lower effective as an anxiolytic drug and better effect as a sedative and hypnotic. The improvement in locomotor activity when compared with diazepam shows this drug is better than Diazepam.
Acknowledgement
Authors are grateful to their respective institutions, where we are working, and Manonmaniam Sundaranar University, Tirunelveli, for extending permission, support and infrastructure.
References
- Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF,Defeo JD, Jones RE, Hartman GD, Huff JR,Duggon ME.Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med ChemLett 2005; 15: 761-764.
- Neugebauer V, Chen PS,Wilgs WD. Role of Metabotropic Glutamate Receptor Subtype mGluR1 in Brief Nociception and Central Sensitization of Primate STT Cells. JNeurophys 1999; 82: 272-282.
- Hrvanitis AG, Gilligan PJG,Chorvat RJ,Cheeseman RSG, Christos TE,Bakthavatchalam R, Beck JP. Non-Peptide Corticotropin-Releasing Hormone Antagonists: Syntheses and Structure−Activity Relationships of 2-Anilinopyrimidines and-triazines. JMedChem 1999; 42: 805-818.
- Dailey S, Feast WJ, Peace RJ, Sage IC, Till S, Wood EL.Synthesis and device characterisation of side-chain polymer electron transport materials for organic semiconductor applications. J Mater Chem 2001; 11: 2238-2243.
- Emanuel S,Gruninger RH, Fuentes-Pesquera A, Connolly PJ,Seamon JA, Hazel S,Tominvich R, Hollister B, Napier C,D’Andrea MR. A Vascular Endothelial Growth Factor Receptor-2 Kinase Inhibitor Potentiates the Activity of the Conventional Chemotherapeutic Agents Paclitaxel and Doxorubicin in TumorXenograft Models. MolPharmacol 2004; 66: 635-647.
- Mirabdolbaghi R, Hassan M, Dudding T. Design and synthesis of a chiral seven-membered ring guanidine organocatalyst applied to asymmetric vinylogousaldol reactions. Tetrahedron: Asymmetry 2015; 26: 560-566.
- Sangshetti JN,Choulte RS,Jadhar MR,Sakle NS,Chabukswar A,Konjari I,Daeandale S,Shinde DB. Green synthesis and anxiolytic activity of some new dibenz-[1,4] diazepine-1-one analogues. Arabian JChem 2013.
- Brown DJ. Diazepines: Supplement II in the Chemistry of tetracyclic compounds, John Wiley & Sons: New Jersey, 2004.
- Robinson RS, Taylor RJK. Quinoxaline Synthesis from α-Hydroxy Ketones via a Tandem Oxidation Process Using Catalysed Aerobic Oxidation.SynLett 2005;6: 1003-1005.
- Wang W,Shen Y,Meng X, Zhao M, Chen Y, Chen B. Copper-Catalyzed Synthesis of Quinoxalines with o-Phenylenediamine and Terminal Alkyne in the Presence of Bases. Org Lett 2011; 13: 4514-4517.
- Breslow R. Determining the Geometries of Transition States by Use of Antihydrophobic Additives in Water. AccChem Res 2004; 37: 471-478.
- Yemitan OK,Salatideen HM. Neurosedative and muscle relaxant activities of aqueous extract of Bryophyllumpinnatum.Fitoterapia 2005; 76: 187-193.
- Roy R, Hofer-BusseTh,Kayser D.New perspective in acute toxicity of chemicals. ToxicolLett 1986.
- Costa-Campos L,Dassoler SC,Rigo AP,Iwu M,Elisabetsky E. Anxiolytic properties of antipsychotic alkaloid alstonine. PharmacolBiochemBehav 2004; 77: 481-489.
- Kalueff AV, Tuohimaa P.Experimental modeling of anxiety and depression. ActaNeurobiolExp 2004; 64: 439-448.
- Achliya GS, Wadodkar SG, Dorle AK. Evaluation of CNS activity of BramhiGhrita. Indian J Pharmacol 2005; 37: 33-36.
- Kulkarni SK. Handbook of Experimental Pharmacology.VallabhPrakashan, Delhi, 1999.
- Xiao-Liang Y, Chun-Me X, Shao-Miao L,Jiu-Xi C, Jin-Chang D, Hua-yue W, Wei-Ke S. Eco-Friendly Synthesis of 2-Substituted BenzothiazolesCatalyzed by Cetyltrimethyl Ammonium Bromide (CTAB) in Water.J BrazChemSoc 2010; 21: 37-42.
- Muhammed N,Saeed M, Khan H,Haq I.Evaluation of n-hexane extract of Viola betonicifolia for its neuropharmacological properties. J NatMed 2013; 67:1-8.
- Pareek A, Kumar N, Agarwal A, Sharma P, Kishore D. 1, 5 Benzodiazepines: Overview of Properties and Synthetic Aspects. ResJ ChemSci 2013; 3: 90-103.
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry 2003; 8: 721-737.
- Jing-Jing C, Jian-Ping Z, Xiang-Qiang P, Zhang W.Gallium(III) triflate-catalyzed synthesis of quinoxaline derivatives. Tetrahedron Lett 2008; 49: 7386-7390.
- Kumarasamy S,Chien-Shu C, Chi-Fen C,Srinivas P,Khagga M, Chi-Rei Wu, Ta-Hsien C.Synthesis, Anticonvulsant, Sedative and Anxiolytic Activities of Novel Annulated Pyrrolo[1,4]benzodiazepines. IntJMolSci 2014; 15: 16500-16510.
- Garcia DA,Busons J, Vale C,Sunol C. Allosteric positive interaction of thymol with the GABAA receptor in primary cultures of mouse cortical neurons. Neuropharmacology 2006; 50: 25-35.