ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 17
High trans fatty acid intake reduces the relative ω-3 fatty acid content in the erythrocyte membrane
Qiang Peng1, Yin Li1, Wu Yang2, Ling Wang1, Ping Li1 and Hai Su1*
1Department of Cardiology, the Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, PR China
2Department of Emergency, Jian Central Hospital, Jian, Jiangxi, PR China
- *Corresponding Author:
- Hai Su
Department of Cardiology
The Second Affiliated Hospital of Nanchang University
Nanchang, Jiangxi, PR China
Received date: August 21, 2017
In this study, the effects of high Trans Fatty Acid (TFA) intake on fatty acid contents in erythrocyte membranes were examined. Thirty-two rabbits were randomly divided into 4 groups: a control group fed a common diet, a high TFA group fed a normal diet supplemented with 5.0 g/kg/d TFA, a High-Fat (HF) group fed a high cholesterol diet, and a TFA+HF group. Saturated FAs, unsaturated FAs and TFA contents of erythrocyte membranes were determined at 0, 4, 8 and 12 weeks. We found that the TFA and saturated FA contents in erythrocyte membranes were significantly higher in the TFA group than in the control group, but ω-3 FAs were lower after 12 weeks. The ω-3/ΣTFA and ω-3/C18:0 ratios were also significantly lower in the TFA group than the control group. The ratios were similar in the HF group and the TFA group. In the TFA+HF group, the abnormalities in the FA ratios in the erythrocyte membrane were more obvious. High TFA intake could decrease polyunsaturated fatty acids in the erythrocyte membrane, especially ω-3 fatty acids, and decrease the ratios of ω-3 to TFA and saturated fatty acids. The effects of high cholesterol intake were equivalent to the effects of high TFA intake. Synergistic effects were clearly observed after combined TFA and high cholesterol intake.
Keywords
Trans fatty acids (TFA), Erythrocyte membrane, Constitution ratios of fatty acids, Rabbit
Introduction
Fatty Acids (FAs) are necessary components of the cell membrane and have a variety of biological activities. Normal lipid content in the cell membrane is important for maintaining normal cell morphology and function. Changes in the ratios of saturated FAs to unsaturated FAs in the cell membrane are closely associated with some diseases [1-3].
For a long time, research on the relationship between lipids and atherosclerosis focused on saturated FAs. Recently, the deleterious effects of Trans Fatty Acids (TFA) and the benefits of ω-FA on the cardiovascular system have attracted much attention [4]. Epidemiological studies have demonstrated that TFA intake is positively correlated with ischemic heart disease and sudden cardiac death [5]. It is currently accepted that TFA is a strong atherogenic factor [6-8].
Some studies had found that the relative FA contents in the erythrocyte membrane were abnormal in cardiovascular patients [4,9]. In patients with acute coronary syndrome, TFA was increased meanwhile ω-3 and ω-6 FA was reduced in the erythrocyte membrane [10]. This result suggested that the FA changes in the erythrocyte membrane may promote atherosclerosis and induce cardiac events. But whether these changes were related to fatty acid intake was not clear now. The aim of this study was to investigate whether high TFA intake induced changes of ω-3FA content in the erythrocyte membrane, and whether its effect was similar in high cholesterol intake.
Materials and Methods
Animals and dietary interventions
Thirty-two male New Zealand rabbits (6 weeks old, 2 kg, provided by the Experimental Animal Center of Nanchang University) were randomly assigned to four groups: control group (C) fed a normal diet, high cholesterol group (HF) fed a high cholesterol diet (2% cholesterol, 7.5% egg yolk powder and 8% lard added to the normal diet), TFA group (TFA) fed a normal diet plus 5.0 g/kg/d TFA from margarine, and high fat +TFA group (HF+TFA) fed a high cholesterol+TFA diet.
Water was provided ad libitum. The guidelines for the ethical care and treatment of animals from the Ministry of Science and Technology of China PR were strictly followed. This study was conducted in accordance with the declaration of Helsinki.
This study was conducted with approval from the Ethics Committee of the Second Affiliated Hospital of Nanchang University (No. 201402142036).
Erythrocyte membrane collection and FA analysis
Fasting blood samples were collected before (0) and at 4, 8, and 12 weeks after dietary intervention. The separated red blood cells were treated by hypotonic haemolysis, and the extracted erythrocyte membranes were stored in liquid nitrogen freezers at -130°C for the fatty acid analysis.
The fatty acids in the erythrocyte membranes were analyzed by gas chromatography (6890N Gas Chromatograph; Agilent, Santa Clara, CA, USA). The laboratory procedure was performed according to published methods [11]. The seven FAs included two saturated FAs, i.e., stearic acid (C18:0) and palmitic acid (C16:0), one mono-unsaturated FA, i.e., oleic acid (C18:1), three polyunsaturated FAs, i.e., linoleic acid (C18:2), arachidonic acid (C20:4), and eicosapentaenoic acid (C20:5), and the total ω-3 FA. Two TFAs were included, trans oleic acid (t-C18:1) and trans palmitoleic acid (t-C16:1). The ratios of various FAs to the total FA content are presented. The ratios ω-3/TFA and ω-3/C18:0 were also calculated to reflect the relative changes in the major FAs, especially ω-3.
Statistical analysis
A statistical analysis was performed using the SPSS13.0 software package. All data are expressed as means ± standard deviation (SD). ANOVA was used to compare FA and TFA levels in erythrocyte membranes among groups. A value of P<0.05 was considered statistically significant.
Results
Relative FA contents
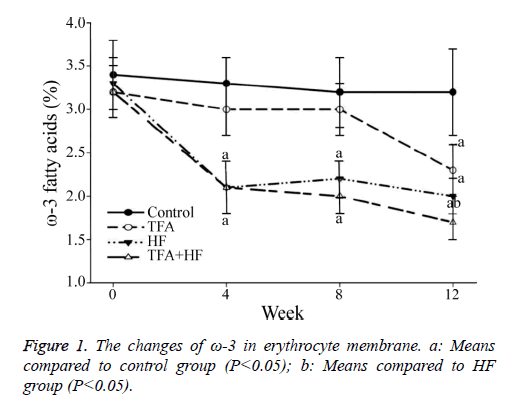
At baseline, similar relative contents of the six FAs and ω-3 were found among the four groups. At week 12, the relative ω-3 FA content was significantly lower in the TFA group than in the control group.
The percentage of FAs that were ω-3 FA was similarly significantly lower in the TFA group than in the HF group at week 12, but the decrease occurred later than in the HF group.
Furthermore, the percentage of FAs that were ω-3 was the lowest in the TFA+HF group (Figure 1). The relative C18:0 content increased and C20:4 and C20:5 decreased in the three interventional groups.
However, the C16:0, C18:1 and C18:2 contents in the three interventional groups did not change significantly during the 12-week period (Table 1).
| Group | Week | ω-3 acids | Saturated FA | Unsaturated FA | ||||
|---|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | C20:4 | C20:5 | |||
| Control | 0 | 3.4 ± 0.4 | 26.3 ± 2.5 | 15.6 ± 1.8 | 13.2 ± 1.7 | 29.2 ± 3.7 | 6.4 ± 0.7 | 3.5 ± 0.2 |
| 4 | 3.3 ± 0.3 | 25.1 ± 2.2 | 14.5 ± 1.7 | 13.0 ± 1.2 | 28.5 ± 3.0 | 6.3 ± 0.9 | 3.4 ± 0.2 | |
| 8 | 3.2 ± 0.4 | 26.5 ± 2.7 | 15.3 ± 1.4 | 12.7 ± 1.6 | 28.4 ± 3.1 | 5.4 ± 0.6 | 3.3 ± 0.2 | |
| 12 | 3.2 ± 0.5 | 27.2 ± 2.7 | 14.9 ± 1.8 | 12.6 ± 1.4 | 29.7 ± 3.3 | 6.3 ± 0.9 | 3.4 ± 0.3 | |
| TFA | 0 | 3.2 ± 0.3 | 26.1 ± 2.7 | 14.1 ± 2.1 | 12.9 ± 1.8 | 28.4 ± 3.5 | 6.4 ± 0.9 | 3.4 ± 0.3 |
| 4 | 3.0 ± 0.3 | 26.6 ± 2.2 | 16.1 ± 2.2 | 13.2 ± 1.4 | 28.5 ± 3.1 | 3.5 ± 0.7a | 2.7 ± 0.2 | |
| 8 | 3.0 ± 0.3 | 25.4 ± 2.4 | 19.7 ± 1.9a | 13.1 ± 1.4 | 29.7 ± 2.7 | 3.5 ± 0.6a | 1.5 ± 0.2a | |
| 12 | 2.3 ± 0.3a | 26.5 ± 2.9 | 19.4 ± 2.0a | 13.7 ± 1.6 | 29.4 ± 2.5 | 3.6 ± 0.7a | 1.4 ± 0.2a | |
| HF | 0 | 3.3 ± 0.3 | 26.9 ± 2.5 | 14.3 ± 1.8 | 12.7 ± 1.6 | 28.7 ± 2.4 | 6.3 ± 0.5 | 3.5 ± 0.3 |
| 4 | 2.1 ± 0.3a | 26.9 ± 2.7 | 17.6 ± 2.7a | 13.0 ± 1.5 | 29.3 ± 2.5 | 4.9 ± 0.6a | 3.1 ± 0.2 | |
| 8 | 2.2 ± 0.2a | 26.6 ± 3.0 | 20.6 ± 2.1a | 14.2 ± 1.7 | 29.1 ± 2.3 | 3.3 ± 0.5a | 1.6 ± 0.2a | |
| 12 | 2.0 ± 0.2a | 25.9 ± 3.0 | 21.1 ± 2.1a | 13.5 ± 1.4 | 29.2 ± 3.3 | 3.3 ± 0.7a | 1.2 ± 0.2a | |
| TFA+HF | 0 | 3.2 ± 0.3 | 26.9 ± 3.0 | 14.2 ± 1.3 | 12.8 ± 1.6 | 28.1 ± 2.6 | 6.3 ± 0.4 | 3.5 ± 0.2 |
| 4 | 2.1 ± 0.3a | 27.3 ± 2.4 | 22.1 ± 3.2ab | 12.4 ± 1.7 | 28.0 ± 3.1 | 3.3 ± 0.6a | 2.0 ± 0.2a | |
| 8 | 2.0 ± 0.2a | 25.2 ± 2.5 | 24.7 ± 3.1ab | 13.9 ± 1.7 | 29.4 ± 2.7 | 3.2 ± 0.6a | 1.1 ± 0.1ab | |
| 12 | 1.7 ± 0.2ab | 27.5 ± 3.1 | 24.6 ± 2.8ab | 13.8 ± 1.5 | 29.6 ± 3.2 | 2.6 ± 0.5ab | 0.9 ± 0.1ab | |
| C16:0 Palmitic acid; C18:0 Stearic acid; C18:1 Oleic acid; C18:2 Linoleic acid; C20:4 Arachidonic acid; C20:5 Eicosapentaenoic acid. Compared to control group, ameans P<0.05. Compared to HF group, bmeans P<0.05. | ||||||||
Table 1: The percentages of CFA in erythrocyte membrane (%).
The percentages of TFA
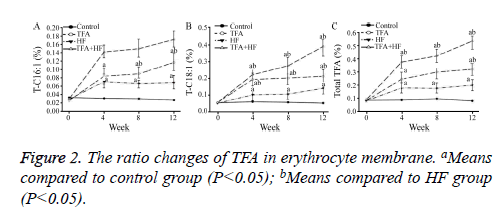
At week 4, the percentages of TFAs that were T-C16:1 and T-C18: 1 and the total TFA content were significantly higher in the three intervention groups than in the control group.
The total TFA content increased as the intervention time increased in the TFA and TFA+HF groups (Table 2). At week 12, the total TFA in the HF, TFA and TFA+HF group are increased compared with the control group (Figure 2).
| Group | Week | T-C16:1 | T-C18:1 | Total TFA |
|---|---|---|---|---|
| Control | 0 | 0.033 ± 0.002 | 0.051 ± 0.004 | 0.084 ± 0.012 |
| 4 | 0.031 ± 0.003 | 0.057 ± 0.006 | 0.086 ± 0.009 | |
| 8 | 0.030 ± 0.004 | 0.054 ± 0.005 | 0.095 ± 0.017 | |
| 12 | 0.027 ± 0.002 | 0.049 ± 0.006 | 0.079 ± 0.013 | |
| TFA | 0 | 0.026 ± 0.004 | 0.052 ± 0.005 | 0.082 ± 0.015 |
| 4 | 0.084 ± 0.019a | 0.189 ± 0.039ab | 0.243 ± 0.045a | |
| 8 | 0.090 ± 0.017ab | 0.197 ± 0.037ab | 0.297 ± 0.051ab | |
| 12 | 0.117 ± 0.020ab | 0.208 ± 0.039ab | 0.319 ± 0.048ab | |
| HF | 0 | 0.029 ± 0.003 | 0.048 ± 0.007 | 0.080 ± 0.016 |
| 4 | 0.071 ± 0.017a | 0.098 ± 0.031a | 0.177 ± 0.040a | |
| 8 | 0.067 ± 0.012a | 0.102 ± 0.029a | 0.174 ± 0.039a | |
| 12 | 0.069 ± 0.016a | 0.137 ± 0.033a | 0.198 ± 0.042a | |
| TFA+HF | 0 | 0.030 ± 0.002 | 0.052 ± 0.004 | 0.088 ± 0.018 |
| 4 | 0.142 ± 0.015ab | 0.217 ± 0.041ab | 0.372 ± 0.047ab | |
| 8 | 0.150 ± 0.022ab | 0.269 ± 0.049ab | 0.419 ± 0.049ab | |
| 12 | 0.172 ± 0.019ab | 0.384 ± 0.057ab | 0.531 ± 0.059ab | |
| T-C16:1 Trans palmitoleic acid; T-C18:1 Trans oleic acid; Compared to control group, ameans P<0.05. Compared to HF group, bmeans P<0.05. | ||||
Table 2: The percentages of TFA in erythrocyte membrane (%).
The ω-3/Σ-TFA and ω-3/C18:0 ratios
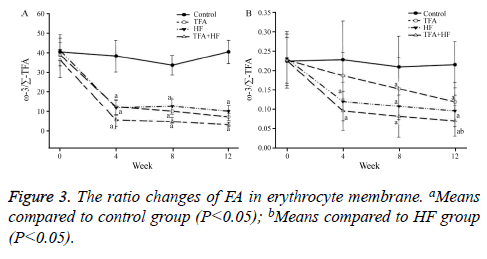
Compared to the control group, the TFA group not only had a significantly lower ω-3/Σ-TFA ratio, but also a lower ω-3/ C18:0 ratio at the 3 time points. The ω-3/Σ-TFA curves of the TFA and HF groups were similar. In the TFA+HF group, the abnormalities in the 2 ratios were the most obvious between the three intervention groups and the control group (Figure 3).
Discussion
Normal lipid content is important for maintaining the normal functions of cells. We know that mature erythrocytes have no nuclei. So erythrocyte membrane was used in some researches to evaluate FA contents in the cell membrane. They have confirmed that abnormal FA contents in erythrocyte membranes, especially abnormalities in TFA and ω-FA, are related to many health problems [3,4,12]. Additionally, ω-3 FA has cardiovascular protective effects; low ω-3 FA intake increases the incidence of arrhythmia and coronary heart disease [3,9]. Some studies have demonstrated that the amount of ω-6/ω-3 in the erythrocyte membrane is negatively correlated with coronary heart disease [13,14].
At present, TFA is a focus of health care. It is widely accepted that high TFA intake increases the risk of coronary death, fatal arrhythmia, and other cardiovascular diseases. Some studies have demonstrated that patients with a higher t-C18:1 content in the erythrocyte membrane have a higher risk of sudden cardiac death [12,15]. Additionally, TFA-induced damage to the cardiovascular system is stronger than damage induced by saturated Fats [15].
The real reasons of FA exchange between the diet and the cell membrane were unknown. Some studies have demonstrated that the FA content of the erythrocyte membrane can reflect the FA content of the cell membranes of other tissues, such as endothelial and myocardial cells [5,15].
Our results showed that high TFA intake not only increases the TFA content in the erythrocyte membrane, but also increases percentage of FAs that are saturated FAs (C18:0). Additionally, a high TFA intake significantly decreased the percentage of FAs that are ω-3 FAs and other unsaturated FAs, especially C20:5. The magnitude of the decrease in unsaturated FAs was similar to that observed after high cholesterol intake. These changes induced significant abnormalities in the relative contents of several FAs in the erythrocyte membrane, such as decreases in the ω-3/C18:0 and C18:1/C18:0 ratios. Abnormal FA ratios may reflect content and functional abnormalities of the cell membrane.
These results also indicated that the abnormal FA contents in the erythrocyte membrane induced by high TFA intake were similar to those induced by high cholesterol intake, particularly the decreases in ω-3 FA and poly-unsaturated FAs.
It is still unclear why a high TFA intake decreases polyunsaturated FAs, especially ω-3 FA, in the erythrocyte membrane. The spatial structure of TFA may be a factor. TFA has a linear spatial structure similar to that of saturated FAs, while the ω-3 FA molecule is bent. A larger repulsive force may exist between TFA and ω-3 FA molecules with different spatial structures and this repulsive force may remove ω-3 FA from the cell membrane, and decrease the ω-3 FA content, but not saturated FA. Another potential mechanism is that TFA may lead to dyslipidaemia by affecting the liver X receptor, cholesterol ester transfer protein, and sterol regulatory element binding protein-1. However, the specific mechanisms are still unknown and worth investigating in future studies.
Our results also indicated that the TFA content increased slightly in the high cholesterol intake group. This may be explained by the lard and egg yolk in the TFA (7.66 and 7.97 mg/g, respectively). In the group with both high TFA and cholesterol intake, the TFA content and the relative contents in the erythrocyte membrane were the highest.
Conclusion
High TFA intake not only induces increases in TFA and saturated fatty acids, but also decreases polyunsaturated fatty acids, especially ω-3 fatty acids, in the erythrocyte membrane. Since FA abnormalities induced by high TFA intake were similar to those induced by high cholesterol intake, TFA may be an atherogenic factor, similar to cholesterol.
Funding
This study was supported by a grant from the Education Research Plan of Jiangxi Provincial Department (GJJ13043).
Acknowledgment
I would like to express my gratitude to all those who have helped me in this work. Especially, I would like to thank Huang Bo and Li Zheng, who kindly gave me a hand during the writing of this paper.
Conflicts of Interest
All of the authors declare that they have no conflicts of interest regarding this paper.
References
- Block RC, Harris WS, Reid KJ, Spertus JA. Omega-6 and trans fatty acids in blood cell membranes: a risk factor for acute coronary syndromes. Am Heart J 2008; 156: 1117-1123.
- Amin AA, Menon RA, Reid KJ, Harris WS, Spertus JA. Acute coronary syndrome patients with depression have low blood cell membrane omega-3 fatty acid levels. Psychosom Med 2008; 70: 856-862.
- Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis 2008; 197: 821-828.
- Lemaitre RN, King IB, Raghunathan TE, Pearce RM, Weinmann S, Knopp RH, Copass MK, Cobb LA, Siscovick DS. Cell membrane trans-fatty acids and the risk of primary cardiac arrest. Circulation 2002; 105: 697-701.
- Su H, Yu JH. Trans-fatty acids and cardiovascular diseases. Zhonghua Xin Xue Guan Bing Za Zhi 2007; 35: 586-588.
- Mozaffarian D, Aro A, Willett WC. Health effects of trans-fatty acids: experimental and observational evidence. E J Clin Nutr 2009; 63: 5-21.
- Kleber ME, Delgado GE, Lorkowski S, März W, von Schacky C. Trans-fatty acids and mortality in patients referred for coronary angiography: the Ludwigshafen Risk and Cardiovascular Health Study. Eur Heart J 2016; 37: 1072-1078.
- Iqbal MP. Trans fatty acids-A risk factor for cardiovascular disease. Pak J Med Sci 2014; 30: 194-197.
- Nishizaki Y, Shimada K, Daida H. The balance of omega-3 polyunsaturated fatty acids for-reducing residual risks in patients with coronary artery disease. Acta Cardiol 2017; 72: 240-248.
- Ferreri C, Panagiotaki M, Chatgilialoglu C. Trans fatty acids in membranes: the free radical path. Mol Biotechnol 2007; 37: 19-25.
- Baylin A, Kabagambe EK, Ascherio A, Spiegelman D, Campos H. High, 18:2 trans-fatty acids in adipose tissue are associated with increased risk of nonfatal acute myocardial infarction in Costa Rican adults. J Nutr 2003; 133: 1186-1191.
- Woodside JV, McKinley MC, Young IS. Saturated and trans fatty acids and coronary heart disease. Curr Atheroscler Rep 2008; 10: 460-466.
- Stanley JC, Elsom RL, Calder PC, Griffin BA, Harris WS, Jebb SA, Lovegrove JA, Moore CS, Riemersma RA, Sanders TA. UK Food Standards Agency Workshop Report: the effects of the dietary n-6:n-3 fatty acid ratio on cardiovascular health. Br J Nutr 2007; 98: 1305-1310.
- Harris WS. The omega-6/omega-3 ratio and cardiovascular disease risk: uses and abuses. Curr Atheroscler Rep 2006; 8: 453-459.
- Anderson SG, Sanders TA, Cruickshank JK. Plasma fatty acid composition as a predictor of arterial stiffness and mortality. Hypertension 2009; 53: 839-845.