ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2023) Volume 34, Issue 1
IL-17A promotes the malignant progression of colon cancer cells by activating JAK2/STAT3 signaling pathway.
Hairong Zhou, Junbin Wang, Zhenyuan Gao, Xiao Wu, Yaping Wang, Lu Zhang*
Department of Medical Oncology, The First Affiliated Hospital of Bengbu Medical College, Bengbu City, Anhui Province 233099, China
- Corresponding Author:
- Lu Zhang
Department of Medical Oncology
The First Affiliated Hospital of Bengbu Medical College
Bengbu City
Anhui Province 233099
China
Accepted date: February 16, 2023
Background: Colon Cancer (CC) is a malignant disease of the digestive system. Multiple cancers are caused by IL-17A, which plays a significant role in inflammation. In this paper, the expression of IL-17A in CC patients is discussed, as well as its regulatory role in CC cells.
Methods: Firstly, the IL-17A expression level in the tissues of CC patients was detected by immunohistochemistry, Western blot and RT-qPCR. The IL-17A expression in serum of CC patients was detected by ELISA. Then two colon cancer cell lines HCT116 and HT29 were selected and IL-17A was applied at different concentrations to the cells. Subsequently, CCK8, colony formation assay, flow cytometry and transwell assay were used to detect cell proliferation, cell cycle, invasion and migration. Western blot was used to detect the expression of JAK2/STAT3 signaling pathway-related proteins. Finally, further discussion of the mechanism was conducted with the addition of the JAK2/STAT3 pathway inhibitor AG490.
Results: CC cell proliferation, invasion and migration induced by IL-17A significantly increased. At this point, the JAK2/STAT3 signaling pathway is activated. AG490 can significantly reverse the promoting effect of IL-17A on the malignant progression of CC cells.
Conclusion: IL-17A promoted the malignant progression of colon cancer cells by activating JAK2/STAT3 signaling pathway.
Keywords
IL-17A, Colon cancer, Malignant progression, JAK2/STAT3, Signaling pathway.
Introduction
Clinically, among the most common malignant tumors of the digestive system, Colon Cancer (CC) is the most common form. In the past 30 years, people's living standards are improving, and diets are changing, resulting in the rise of CC from sixth place ten years ago to fourth place today in the world. People's lives and health are seriously threatened by CC [1,2]. Studying the pathogenesis of CC is therefore extremely important.
A member of the Interleukin(IL)-17 family, IL-17A plays a crucial role in inflammation and there is a close relationship between IL-17A in the body and immune function [3]. In addition to being found in a variety of tumor cells, IL-17A has an important regulatory role in cancer development and occurrence [4-6]. IL-17A is released by tumor-associated neutrophils and promotes the migration and invasion of gastric cancer cells [7]. Angiogenesis and tumor growth are enhanced by IL-17A in lung cancer through synergistic action with immune macrophages [8]. The expression of IL-4 and IL-17A has been shown to be significantly increased in human pancreatic cancer cells and synergistically promotes the production of hydrogen peroxide, causes DNA oxidative damage and increases the expression of dual oxidase 2 [9]. There is also a complex link between IL-17A and CC. IL-17A promotes the cell cycle progression and tumor development of CC cells by membrane-bound form [10]. Sinda Bedoui et al. showed differences in the polypeptide variation of the IL-17A gene and CC susceptibility, suggesting that the IL-17A haplotype and variation may serve as potential therapeutic targets for CC [11]. Although studies on IL-17A in CC have increased in recent years, the regulatory mechanism has not been reported in detail.
Therefore, here, we discuss IL-17A's role in CC and how it works as a regulator, to provide a theory of targeted therapy for CC.
Materials and Methods
Tissues
Colorectal cancer tissues were obtained from patients at the First Affiliated Hospital of Bengbu Medical College. All the patients in the trial signed their consent forms, and all procedures were authorized by the Ethical Committee of the First Affiliated Hospital of Bengbu Medical College.
Immunohistochemistry
Paraffin-embedded CC tissues were de-paraffinized in xylene. The slides were blocked using 3% H2O2 for 15 min and then after blocking with 3% BSA for 60 minutes, the slides were immunostained overnight at 4°C with corresponding antibodies. The section was incubated with a secondary antibody the following day (1:1000 dilution; Abcam, CA, USA) and detected through a light microscope.
Cell culture
Colorectal mucosal cells FHC cells and colorectal cancer cells HT29 and HCT116 obtained from Bena Culture Collection (Hebei China) were cultured in RPMI 1640 medium with 10% FBS at 37°C in 5% CO2. Colorectal cancer cells were induced with 0, 5, 10, and 20 ng/ml IL-17A (HY-P7372, MedChemExpress) for 24h, 48h, and 72h. JAK/STAT3 inhibitor AG490 (HY-12000, MedChemExpress) was used to induce the cell for 24h.
RT-qPCR
Trizol was used to extract RNA from patient tumor tissue or HT29 and HCT116 cells. cDNA was synthesized using the Reverse Transcription Kit. The quantitative real-time PCR (qPCR) was operated with a Roche 480. A comparative 2-ΔΔCT was used to determine relative gene expression [12].
Western blot
Total protein from patient tumor tissue or HT29 and HCT116 cells was isolated with lysis buffer (Beyotime). The BCA protein assay kit was then used to measure the protein concentrations. We separated the proteins using SDS-PAGE and transferred them to PVDF membranes (Millipore). 5% skimmed milk was used to block the membranes for 1 hour, followed by incubation with the primary antibodies at 4°C for one night. The next day, the membranes were incubated using secondary antibodies and visualized with an ECL kit, and analyzed using imaging software.
CCK8
HT29 and HCT116 cells were added into a 96-well plate (1×104 cells per well). After giving the corresponding treatment, each well added 10 μl of CCK8 solution (C0038; Beyotime) for 4h and then measured the absorbance at 450 nm with a microplate reader.
Colony formation assay
HT29 and HCT116 cells were put in in 6-well plates (5000 cells per well) and then induced with IL-17A at a concentration of 10 ng/ml. The cells were incubated for 14 days and then fixed with methanol, and stained with a 0.1% crystal violet solution for 30 minutes.
Cell cycle analysis assay
Cells were put in 6-well plates (1 × 106 per well). Cells were fixed with 75% ethanol overnight at -20°C. The next day, a total of 450 μl PBS and 50 μl propidium iodide at a concentration of 0.5 mg/ml was added to each tube. The cell cycle was analyzed by flow cytometer.
Transwell assay for migration and invasion
The treated HT29 and HCT116 cells were suspended (2.5 × 105 per well) in RPMI-1640 medium. Matrigel (BD Biosciences) was used for invasion assays. A 100 μl aliquot of the mixture was added to the upper chambers and incubated at 37°C for 5 h to solidify. 200 μl cell suspensions were added to the upper chamber, and 700 μl of cultured medium containing 10% FBS was added to the lower chamber. Fixation and staining of migrated cells with 4% paraformaldehyde and 0.1% crystal violet were performed for 20 minutes at room temperature after 24 h. Subsequently, the cells selected from five random fields were counted with a light microscope.
Statistical analysis
Statistical significance between two groups was determined with a student’s t-test, and statistical significance between multiple groups was determined with one-way ANOVA using GraphPad Prism software version 8. Data were presented as means ± SD. P<0.05 was considered to be statistically significant.
Results
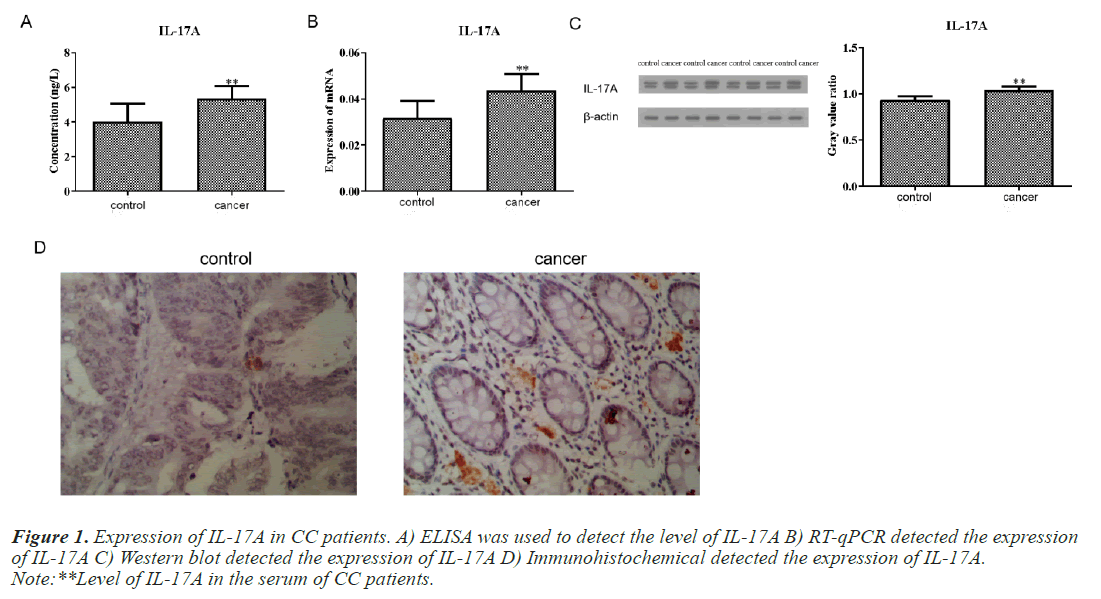
Expression of IL-17A in CC patients
We first detected the level of IL-17A in the serum of CC patients and normal people. The results of ELISA test showed that the content of IL-17A in the serum of CC patients was significantly higher than that of normal people (Figure 1A). Subsequently, we also detected the protein level and mRNA level of IL-17A in tumor tissue and paracancerous tissue of CC patients and found that the expression of IL-17A in tumor tissue was significantly higher than that in paracancerous tissue (Figures 1B and 1C). This is consistent with the serum ELISA results of CC patients. Immunohistochemical experiments further confirmed this result (Figure 1D).
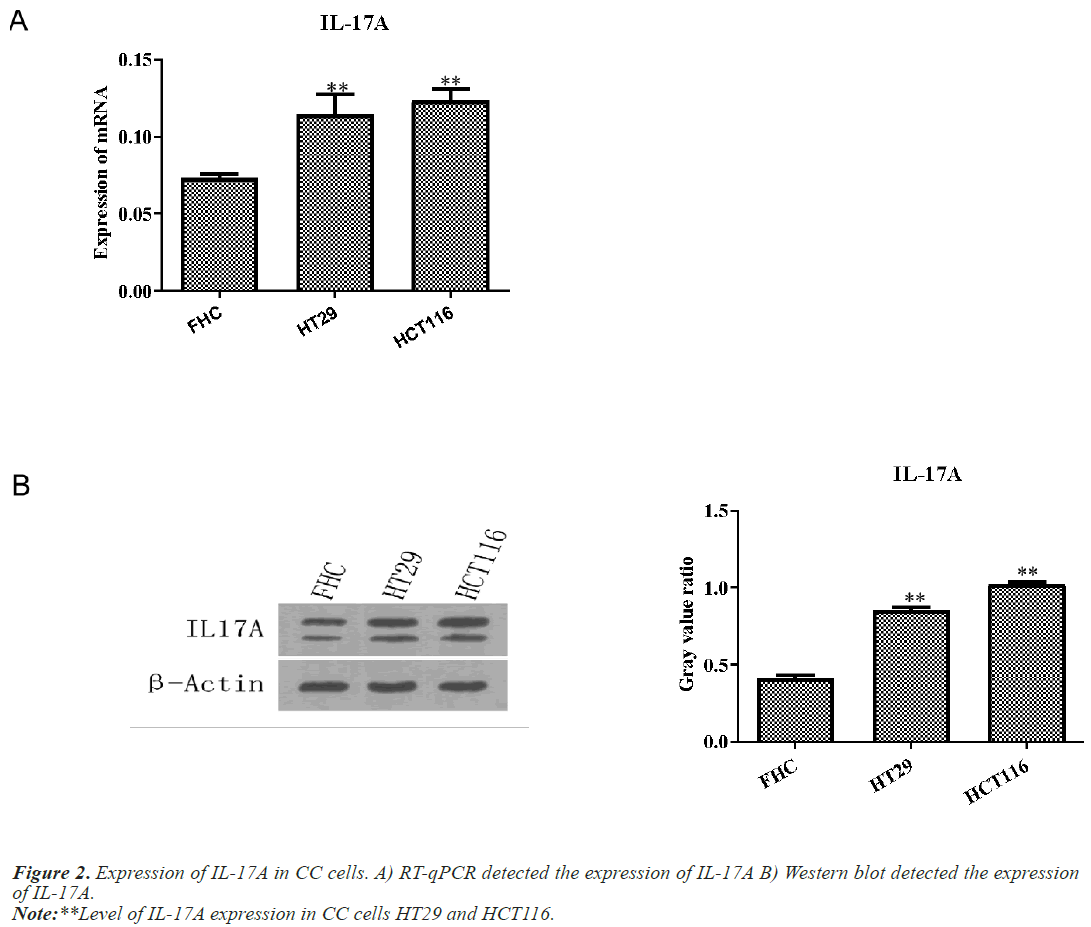
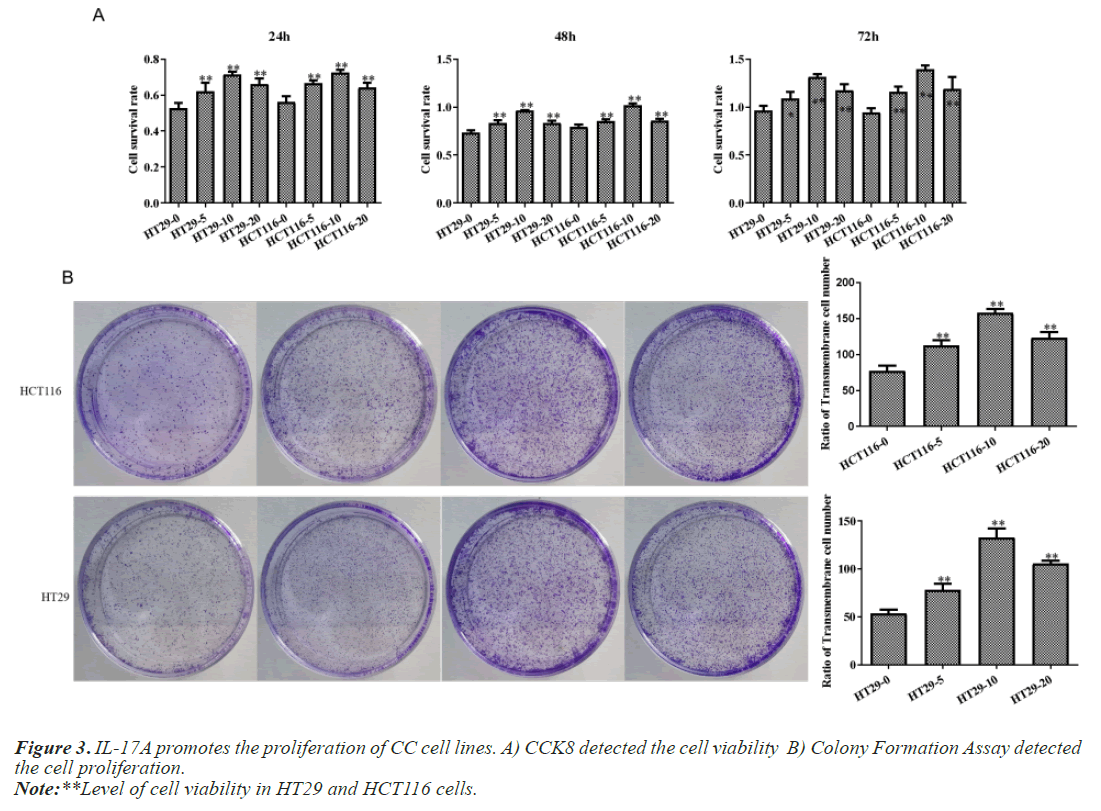
IL-17A promotes the proliferation, migration and invasion of CC cells
Next, we examined the expression of IL-17A in CC cells and normal colon cells FHC. Western blot and RT-qPCR results showed that IL-17A expression in CC cells HT29 and HCT116 was significantly increased compared with normal colon cell FHC (Figure 2).
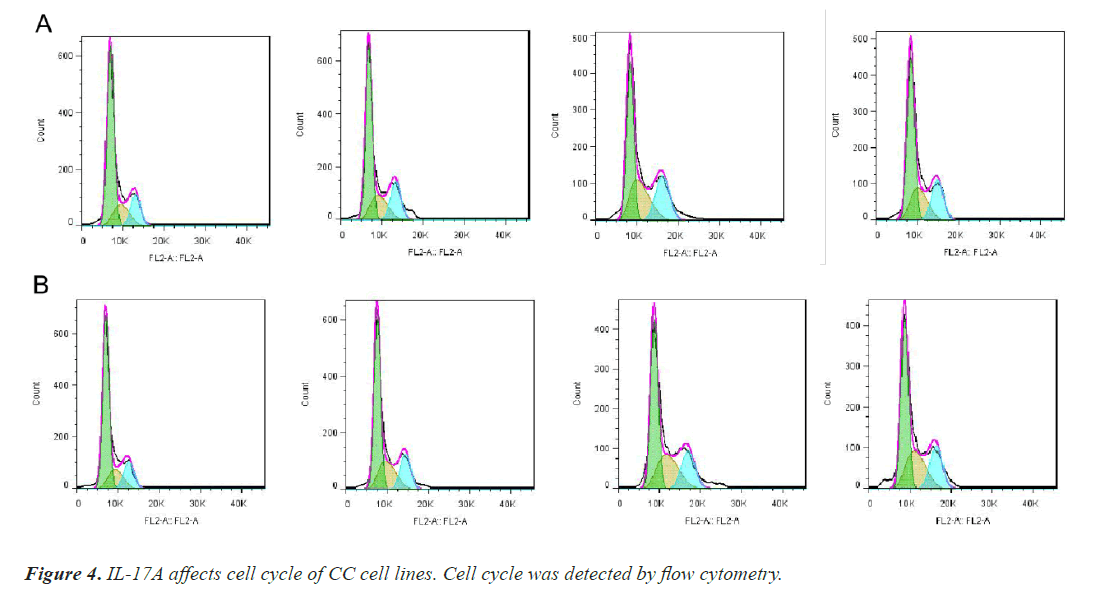
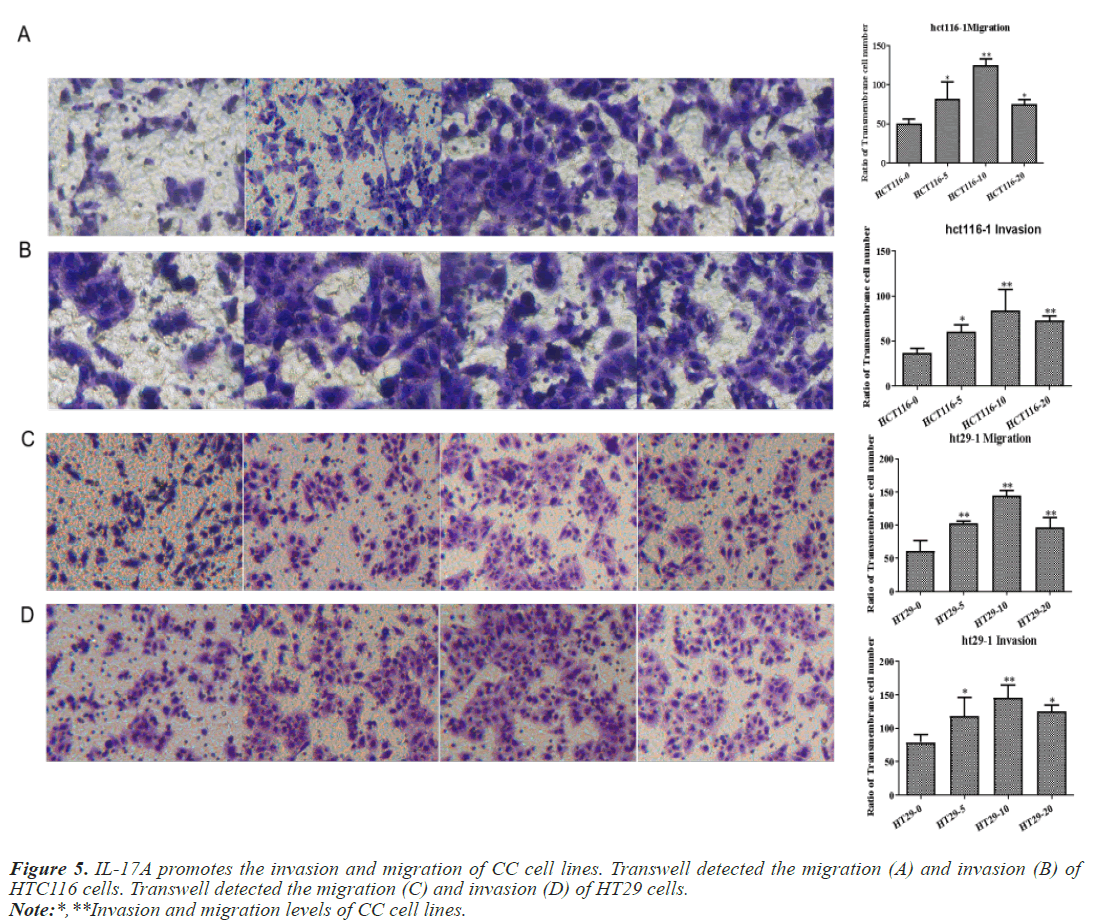
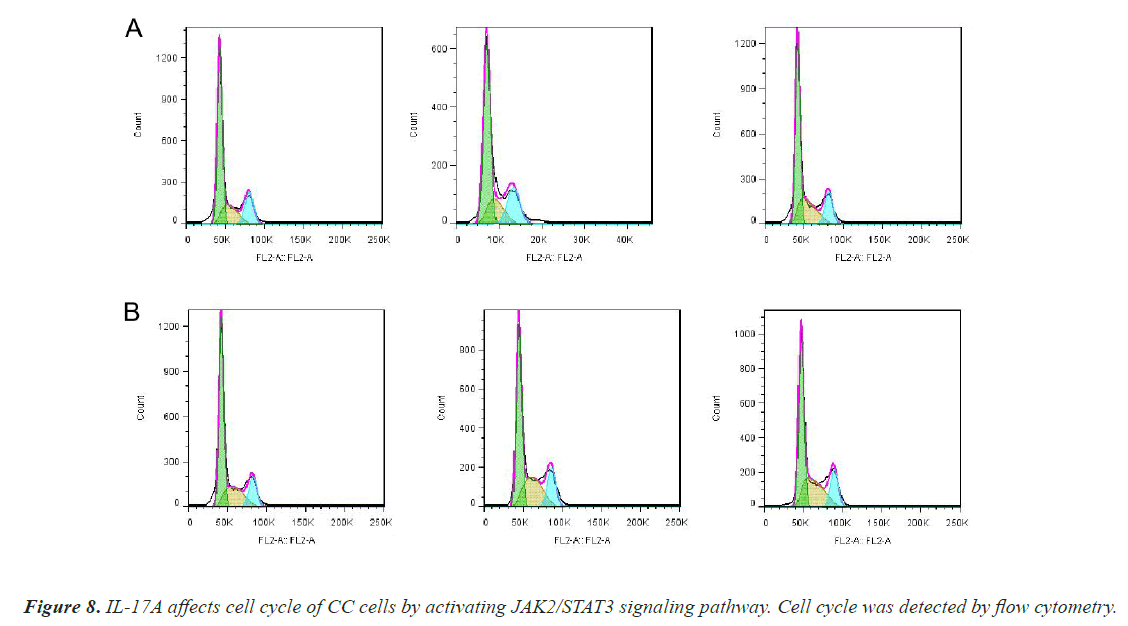
After that, CC cells were stimulated with IL-17A at 0, 5, 10, and 20ng/ml, respectively. The results of the CCK8 experiment showed that IL-17A significantly promoted the cell viability of HT29 and HCT116 cells (Figure 3A). Clonogenic assays were also used to examine the effect of IL-17A on the proliferation of HT29 and HCT116 cells and found that IL-17A significantly promotes the clonogenicity of HT29 and HCT116 cells (Figure 3B). We also examined the effect of IL-17A on the cell cycle and found that the cell G1 phase was significantly decreased, while the S and G2 phases were significantly increased (Figure 4), and cell invasion and migration were significantly increased (Figure 5) after induction of 5 and 10 ng/ml IL-17A. Compared with the 10 ng/mL IL-17A induction group, the proliferation, cycle, invasion and migration of cells showed a downward trend after 20 ng/ mL IL-17A induction.
IL-17A activates the JAK2/STAT3 signaling pathway
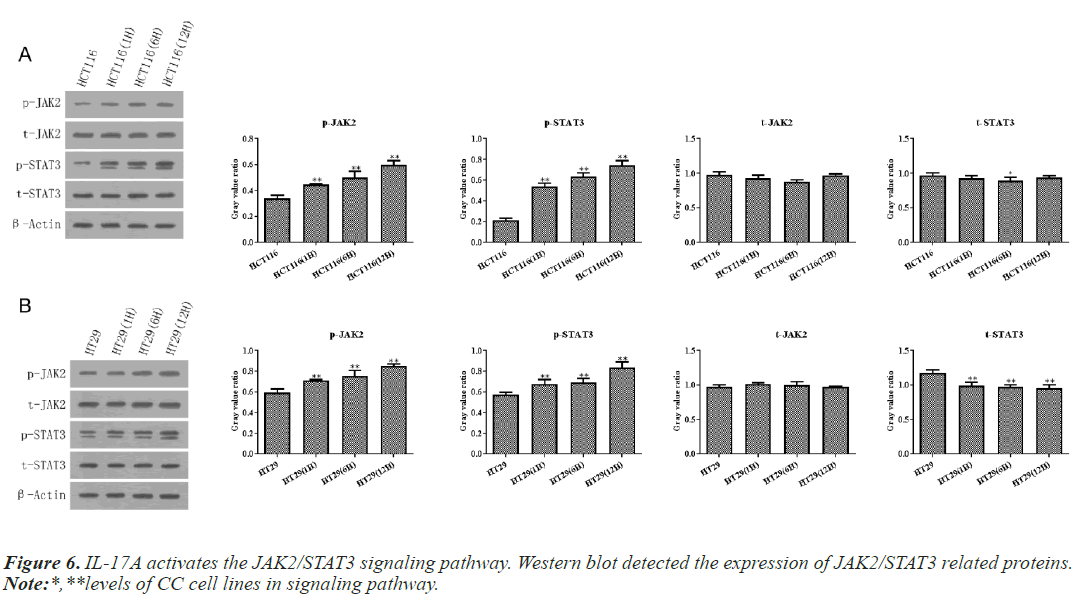
According to the above experiments, we selected 10ng/ ml IL-17A to stimulate HT29 and HCT116 cells for 0, 6, and 12h in the following experiments. Then western blot and RT-qPCR assay showed that IL-17A did not affect the expression of JAK2 and STAT3, but significantly promoted the phosphorylation levels of JAK2 and STAT3 (Figure 6), indicating that the JAK2/STAT3 signaling pathway was activated after IL-17A induced HT29 and HCT116 cells.
IL-17A affects the biological function of CC cells through JAK2/STAT3 signaling pathway
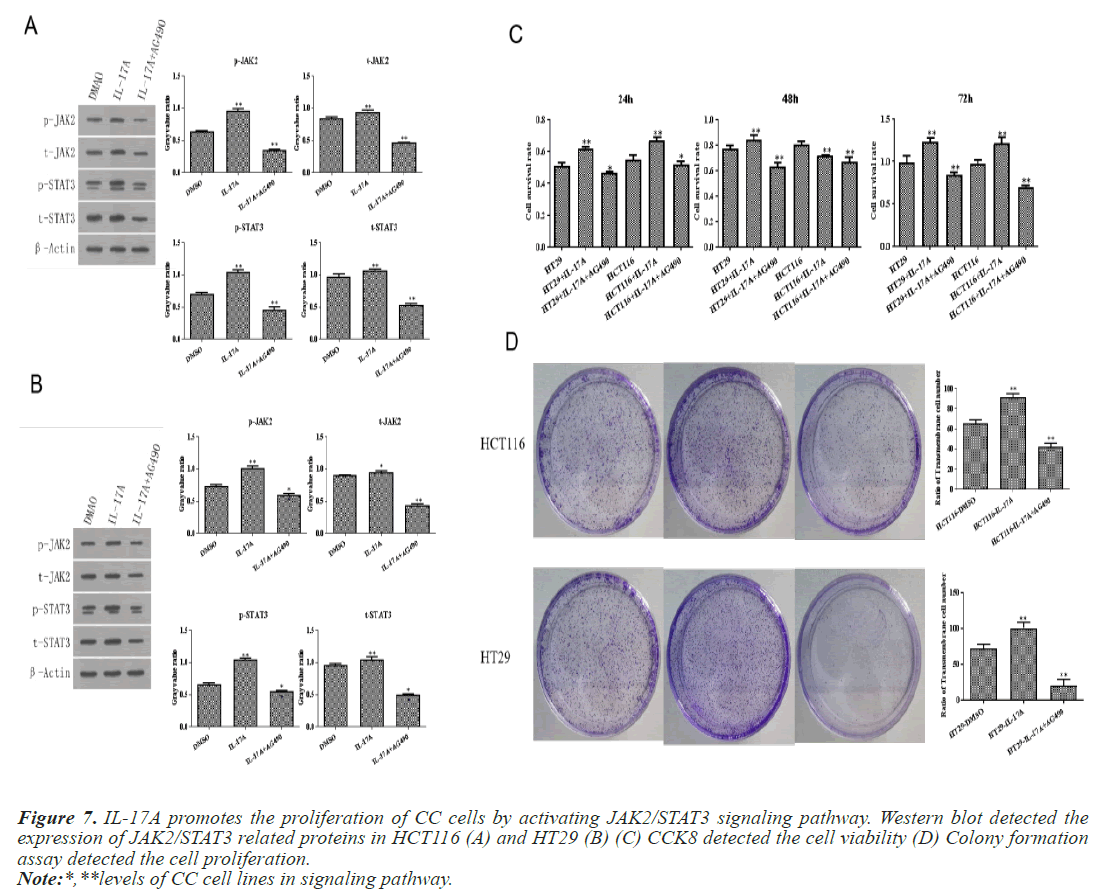
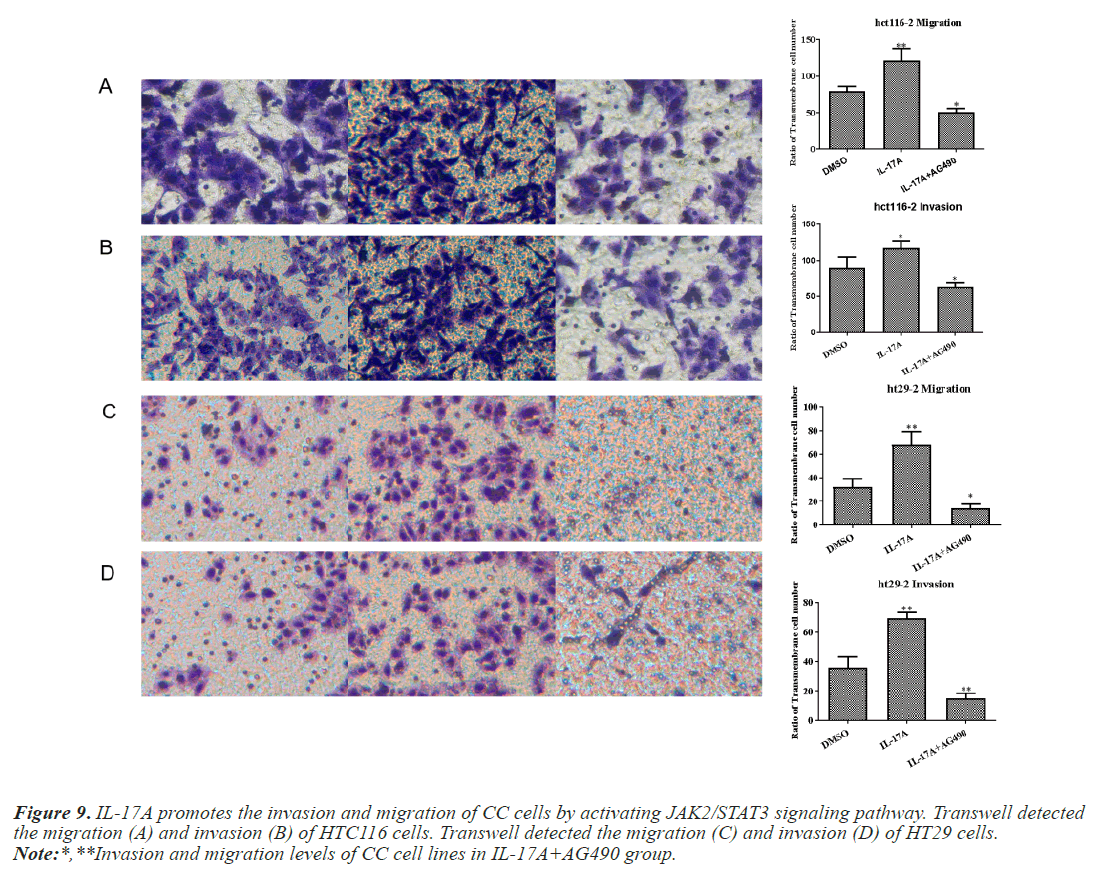
To investigate whether IL-17A affects CC through the JAK2/STAT3 signaling pathway, we administered inhibitors of JAK2/STAT3 signaling pathway, AG490, and divided the cells into control group, IL-17A group, and IL-17A+AG490 group. Western blot analysis of the pathway showed that compared with IL-17A, additional administration of AG490 significantly inhibited the phosphorylation of JAK2 and STAT3, indicating that the pathway was successfully inhibited (Figures 7A and 7B). CCK8 and colony formation assay results showed that compared with IL-17A, cell proliferation ability in IL- 17A+AG490 group was significantly decreased (Figures 7C and 7D). At the same time, we also found that compared with IL-17A, the G1 phase in IL-17A+AG490 group was significantly increased, while the S and G2 phases were significantly decreased, indicating cell cycle arrest (Figure 8). Transwell results showed that invasion and migration were significantly reduced in the IL-17A+AG490 group compared with IL-17A (Figure 9). These results suggest that IL-17A affects the biological function of CC cells through JAK2/STAT3 signaling pathway.
Figure 7: IL-17A promotes the proliferation of CC cells by activating JAK2/STAT3 signaling pathway. Western blot detected the expression of JAK2/STAT3 related proteins in HCT116 (A) and HT29 (B) (C) CCK8 detected the cell viability (D) Colony formation assay detected the cell proliferation.
Note:*,**levels of CC cell lines in signaling pathway.
Figure 9: IL-17A promotes the invasion and migration of CC cells by activating JAK2/STAT3 signaling pathway. Transwell detected the migration (A) and invasion (B) of HTC116 cells. Transwell detected the migration (C) and invasion (D) of HT29 cells.
Note:*,**Invasion and migration levels of CC cell lines in IL-17A+AG490 group.
Discussion
CC doesn't show obvious symptoms in the early stages, once diagnosed, it is in the middle to advanced stages for most of them, and only about 50% of people survive five years [13]. Due to the lack of timely detection, the treatment of CC often requires the combined application of surgery, radiotherapy, and chemotherapy. After diagnosis, most patients will choose surgical treatment, and surgical treatment has become the main means of CC cancer treatment [14]. However, the possibility of recurrence and metastasis with surgery and other treatments makes the treatment of CC more difficult. It is important to elucidate the mechanism of CC for treatment or prevention.
CC results from abnormal gene expression in multiple genes. In order to control tumor growth, one of the most effective methods is to regulate certain genes in the process of tumor development. A previous study has shown that early colonic lesions of the mouse model of sporadic CC show massive expression of IL-17A [15]. The probability of colitis-related cancer in IL-17A deficient mice is lower than that in wild-type mice. Inhibiting IL-17A in mice can significantly inhibit the occurrence and proliferation of tumor in mice [16]. In our experiment, we proved that the expression of IL-17A was abnormally elevated in tissues and blood of CC patients. Although there have been reports on the role of IL-17A in CC, its specific regulatory mechanism and its expression in clinical CC patients have not been reported. So next, we explored the effect of IL- 17A on CC cells in cell experiments. We selected HT29 and HCT116, two common CC cell lines, to make our results more comprehensive and convincing. We found that cell proliferation, cell cycle, invasion and migration were significantly increased in IL-17A-stimulated HT29 and HCT116 cells.
Next, we further discussed the downstream regulatory pathway of IL-17A. Through literature review, we found that in gastric cancer, a pathway involving IL-17A/JAK2/ STAT3 promotes the migration and invasion of gastric cancer cells by cancer-associated fibroblasts [17]. As a result of its activation of JAK2/STAT3, IL-17A induces osteoblast differentiation in ankylosing spondylitis [18]. Through activation of the JAK2/STAT3 pathway, IL-17A promotes the progression of diffuse large B-cell lymphomas [19]. Further, a decrease in JAK2/STAT3 pathway activity may also induce apoptosis in CC cells [20]. Moreover, the regulation of JAK2/STAT3 pathway can significantly affect the proliferation and cycle progression of CC cells [21]. JAK2/STAT3 appears to play an important role in the development and progression of CC. However, no studies have reported whether IL-17A regulates the JAK2/STAT3 pathway, which leads to CC occurrence and development.
In our experiment, JAK2/STAT3 signaling was found to be activated by IL-17A in HT29 and HCT116 cells. To further explore the mechanism, AG490, an inhibitor of JAK2/STAT3 signaling, significantly reversed IL-17A's pro-proliferation, cycle, invasion, and migration effects. These results suggest that IL-17A promotes the malignant progression of CC cells by activating JAK2/STAT3 signaling pathway.
Conclusion
Overall, we conclude that IL-17A promotes the malignant progression of CC cells through JAK2/STAT3 signaling pathway. As a result of our paper, targeted therapy for CC has gained theoretical support.
Funding
The research was supported by Key project of Natural Science, Bengbu Medical College [2020byzd144].