ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 2
Immunogenic chemotherapy sensitizes non-small cell lung cancer to immune checkpoint blockade therapy in preclinical models
1Department of Cardiothoracic Surgery, Dongguan Third People’s Hospital, Dongguan, Guangdong, PR China
2Information Section, Dongguan Third People’s Hospital, Dongguan, Guangdong, PR China
3Department of Thoracic Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Hubei, Wuhan, PR China
- *Corresponding Author:
- Jun Lu
Department of Cardiothoracic Surgery
Dongguan Third People’s Hospital
Guangdong, PR China
Accepted date: July 01, 2016
Background: Lung cancer is the top common cancer and cause of cancer-related death worldwide. Checkpoint blockade immunotherapies have shown extraordinarily anti-tumor effects, but only benefit the minority of patients whose tumors are pre-infiltrated by CD8+ T cells. However, the majority of solid tumors, including lung cancer are not immunogenic and CD8+ T cell infiltration is inconspicuous.
Purpose: We aimed to explore the potential favourable roles of chemotherapy in promoting immune checkpoint blockade therapy in non-small cell lung cancer (NSCLC).
Method: We combined chemotherapy gemcitabine with immune checkpoint blockade therapy anti-PD-1 antibody in preclinical models of NSCLC. Survival analysis and potential mechanisms were also analysed.
Result: We found that lung cancer lacks CD8+ T cell infiltration and resisted to anti-PD-1 antibody when used alone. When combined with chemotherapy drug gemcitabine, more CD8+ T cell infiltration was seen and effects of anti-PD-1 antibody were significantly enhanced. Animals in the combination therapy group showed longer survival time than gemcitabine or anti-PD-1 antibody therapy groups.
Conclusion: In conclusion, our findings suggested that chemotherapy stimulated immunogenic potential of NSCLC and synergized with immune checkpoint blockade therapy.
Keywords
Anti-PD-1, Combination therapy, Immune checkpoint, Non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer death among both men and women in the United States [1,2]. It is also the top reason for male and second reason for female cancer death worldwide [1,2]. Non-small-cell lung carcinoma (NSCLC) is any epithelial lung cancer other than small-cell lung carcinoma (SCLC). NSCLC is the primary histological type which accounts for about 85% of all lung cancers. As a class, NSCLCs are relatively insensitive to chemotherapy, compared to small cell carcinoma. Treatment for advanced NSCLC remains an unmet need; little chemotherapeutic progress has been made since the approval of docetaxel for second-line treatment [3,4]. Target therapies, such as epidermal growth factor receptor (EGFR) inhibitor have made mild progression in advanced EGFR mutation-positive NSCLC treatment [5]. Therefore, novel treatments are highly needed to improve the overall survival of NSCLC patients.
The functional immune system relies on coordinative work of different immune cell types and regulatory molecules. Immune checkpoints are molecules that either turn up a signal (costimulatory molecules) or turn down a signal (inhibitory molecules) in immune activity [6,7]. The inhibitory signals play critical roles in maintaining self-tolerance, preventing autoimmunity and protecting tissues from immune collateral damage. Tumors are societies of tumor cells and surround stromal cells, including fibroblasts and immune cells which have critical roles in modulating tumor development [8-10]. In tumor immunity, increased expression of inhibitory immune checkpoints programmed death-1 (PD-1)/PD-ligand 1 (PD-L1) and PD-L2 axis and cytotoxic lymphocyte antigen (CTLA-4)/ CD28 system depressed CD8+ T cell activity leading to tumor progression and treatment failure [11,12]. Thus, refining T cell immune response in tumor microenvironment could be a potential way to control tumor growth and eliminate tumors.
So far, three checkpoint inhibitors have been actively explored clinically, including antibodies (Abs) to CTLA-4, PD-1, and the checkpoint ligand PD-L1 [13-15]. Administration of these immune checkpoint blockades has resulted in more than three years extended survival time in around 20% advanced melanoma patients [16,17]. What’s more, a combination of conventional chemotherapies was considered as a synergistic approach of immune checkpoint blockades in various tumors [18]. However, whether the combination of chemotherapy and immune checkpoint blockades can benefit NSCLC patients is still unclear. Here, we conducted a preclinical test aimed to evaluate the potential therapeutic effects of combining chemotherapy with immune checkpoint blockades in NSCLC models.
Materials and Methods
Cell culture
Two mouse lung cancer cell lines (KLN205 and CMT64) were obtained from Sigma-Aldrich. Cell lines were cultured in Waymouth's MB 752⁄1 medium with 2 mM Glutamine, 10% Fetal Bovine Serum, and 1% Antibiotic-Antimycotic (Thermo Fisher Scientific, IL, USA). Subculture was performed at 70% confluent of each cell line.
Cell viability assay
Cell viability assay was conducted using CCK-8 kit (Thermo Fisher Scientific, IL, USA) following the manufacturer’s instructions. Specifically, tested cells (2.5 × 103/well) were seeded in 96-well plates in 100 μl culture medium containing gradient concentrations of paclitaxel, gemcitabine, or carboplatin cultured for 48 h. Then 10 μl CCK-8 solution was added to each well for incubation for 1 h at 37°C. After incubation, absorbance was measured by microplate reader at 450 nm.
Enzyme-linked immunosorbent assay
Measurement of high mobility group box 1 (HMGB1), IFN-γ, Granzyme B, and PD-L1 were conducted by enzyme-linked immunosorbent assay (ELISA). The ELISA kit was purchased from LSBio, Abcam (IFN-γ and Granzyme B), and Cell Signaling Technology. All procedures followed the kit’s instruction.
Flow cytometry
Flow cytometry analysis was used to classify CD8+ T cells, regulatory T cells (Treg), and myeloid-derived suppressive cells (MDSCs) in animal samples. Cells were isolated from animal tissues and washed with PBS once. Then red blood cell lysis buffer was used to remove red blood cells. Cells were then washed with PBS once. Cell membrane staining (CD19, CD3, CD4, CD8, and CD25) was performed directly at room temperature for 15 min. After cell membrane staining, cells were either washed with PBS twice for analysis or fixed by fixation buffer for 30 min at room temperature. After fixation, cells were permeabilized for 15 min at room temperature and stained with FOXP3 for 20 min at room temperature. Finally, cells were washed with PBS twice. CD8+ T cells were gated as CD19-, CD3+, CD4-, and CD8+ cells. Treg cells were gated as CD19-, CD3+, CD4+, CD8-, CD25+ and FOXP3+ cells. For MDSCs, CD11b and Gr-1 were stained following cell membrane staining procedure as mentioned above.
Immunofluorescence
Standard Immunofluorescence was conducted to detect CD3 expression in tissues from mouse studies. FFPE tissue sections were firstly deparaffinized by incubating with xylene using three changes for 5 minutes each. Rehydration was then performed in gradient ethanol for 5min each. Heat-induced antigen retrieval was conducted in citric acid buffer (PH=6.0) at 100°C for 15 min. After washed with PBST once, 5% BSA solution was used for incubation. Primary antibody (1:100) was then added and incubated overnight at 4°C. The next day, slides were washed in PBST three times and the secondary antibody conjugated with FITC dye was incubated 1h at room temperature. Finally, DAPI staining was performed right before covering with a cover slide.
Animal study
All animal studies were approved by the Experimental Animal Committee of the Dongguan Third People’s Hospital, Guangdong, China. Lung cancer syngeneic mouse model was established using C57BL/6J immune component mice (7-week old, 22-24 g, Shanghai SLAC Laboratory Animal Center of Chinese Academy of Sciences, China). The C57BL/6J derived lung cancer cell line CMT64 cells (106) were inoculated to the back of each mouse. After tumor development, mice were randomly distributed into each experimental group (n=10 in each group). Tumor growth was checked and recorded every week. Tumor volume was calculated based on length and width: width2 × length × π/6. Excessive ascites, more than 20% body weight loss and other signs of distress will be considered as the end point. Survival of each mouse was recorded for survival analysis.
Statistical analysis
The software used for statistical analysis in this was Graph Pad (CA, USA). One-way ANOVA, t-test, chi-square analysis and Bonferroni’s pairwise comparisons were used to analyze the difference between individual groups. Kaplan-Meier survival analysis was used to plot the survival curves of tumor-burden mice with different treatments. Two-tailed P<0.05 was considered as statistically significant.
Results
Chemotherapeutic drugs paclitaxel, carboplatin, and gemcitabine are effective on NSCLC models
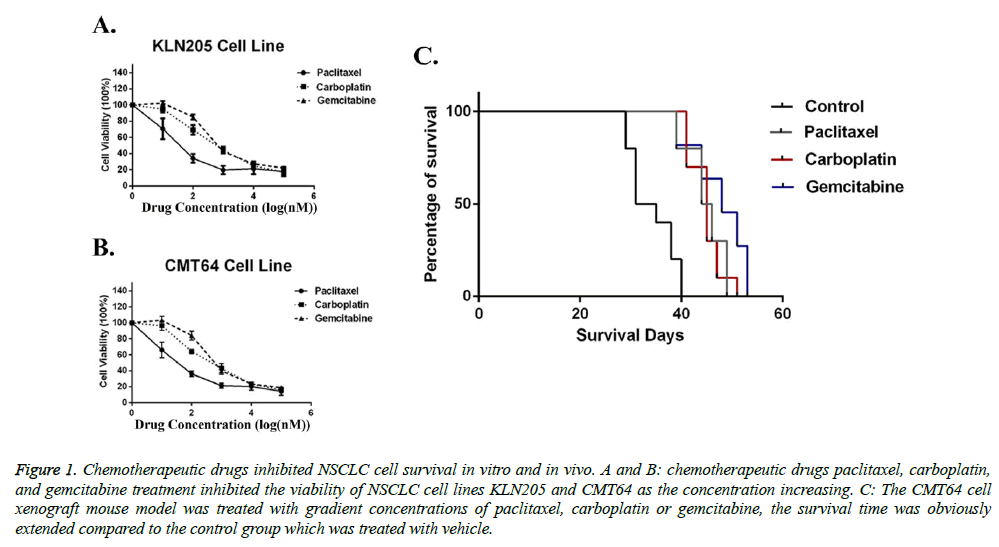
Before investigating the-synergistic role of chemotherapeutic drugs paclitaxel, carboplatin, and gemcitabine to immunotherapy, we first determined their effects in NSCLC models. Two mice-derived NSCLC cell lines (KLN205 and CMT64) were used as target cells. Our ex vivo data showed that all of the three chemotherapeutic drugs can effectively eliminate tumor cells (Figures 1A and 1B). Then we used NSCLC xenograft model to test their in-vivo effects. Compare with saline group, both three drugs (paclitaxel 60 mg/kg b.w., carboplatin 80 mg/kg b.w., and gemcitabine 120 mg/kg b.w.) prolonged survival time of tumor challenging mice (Figure 1C). However, in the xenograft model, no significant difference between three treatments was seen. Taken together, our data indicated that chemotherapeutic drugs paclitaxel, carboplatin, and gemcitabine have similar effects on NSCLC models when used as monotherapy.
Figure 1: Chemotherapeutic drugs inhibited NSCLC cell survival in vitro and in vivo. A and B: chemotherapeutic drugs paclitaxel, carboplatin, and gemcitabine treatment inhibited the viability of NSCLC cell lines KLN205 and CMT64 as the concentration increasing. C: The CMT64 cell xenograft mouse model was treated with gradient concentrations of paclitaxel, carboplatin or gemcitabine, the survival time was obviously extended compared to the control group which was treated with vehicle.
Gemcitabine stimulates tumor immunogenicity of lung cancer cells
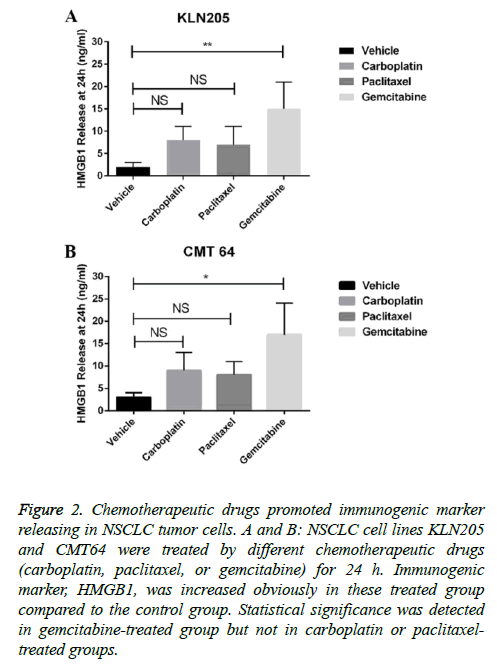
We further investigated the potential of combining these chemotherapeutic drugs with immune checkpoint blockades. The release of HMGB1 was considered as a marker of immune related cell death. By measuring HMGB1 releasing after each drug treatment, we found that gemcitabine was more effective in inducing HMBG1 release than paclitaxel and carboplatin in both two cell lines (Figures 2A and 2B). These data indicated that gemcitabine may enhance immunogenicity of a tumor.
Figure 2: Chemotherapeutic drugs promoted immunogenic marker releasing in NSCLC tumor cells. A and B: NSCLC cell lines KLN205 and CMT64 were treated by different chemotherapeutic drugs (carboplatin, paclitaxel, or gemcitabine) for 24 h. Immunogenic marker, HMGB1, was increased obviously in these treated group compared to the control group. Statistical significance was detected in gemcitabine-treated group but not in carboplatin or paclitaxeltreated groups.
Gemcitabine increases T cell infiltration in lung cancer model
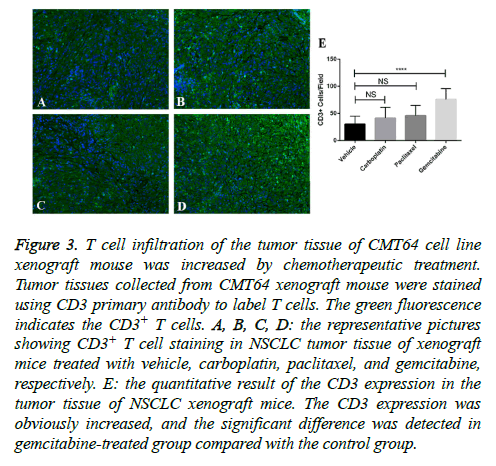
A certain amount of T cell infiltration is the foundation of successful immune checkpoint blockades therapy. We measured T cell infiltration in tumor sections after paclitaxel, carboplatin, and gemcitabine treatments by staining of CD3. We found that small amount of CD3+ cells can be seen in vehicle-treated CMT64 tumors, however much more can be seen in gemcitabine-treated tumors (Figure 3). Other two drugs paclitaxel and carboplatin slight increased CD3+ cell infiltration but didn’t get statistical significance (Figure 3).
Figure 3: T cell infiltration of the tumor tissue of CMT64 cell line xenograft mouse was increased by chemotherapeutic treatment. Tumor tissues collected from CMT64 xenograft mouse were stained using CD3 primary antibody to label T cells. The green fluorescence indicates the CD3+ T cells. A, B, C, D: the representative pictures showing CD3+ T cell staining in NSCLC tumor tissue of xenograft mice treated with vehicle, carboplatin, paclitaxel, and gemcitabine, respectively. E: the quantitative result of the CD3 expression in the tumor tissue of NSCLC xenograft mice. The CD3 expression was obviously increased, and the significant difference was detected in gemcitabine-treated group compared with the control group.
Gemcitabine enhances efficacy of anti-PD-1 in lung cancer model
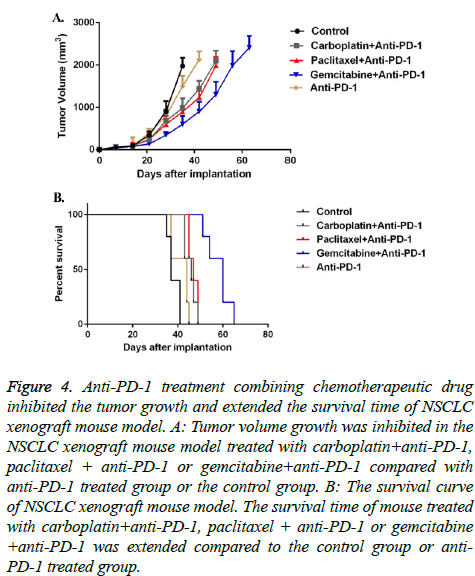
Upon understanding the tumor immunogenic and T cell infiltration roles of different chemotherapeutic drugs, we further combined these chemotherapeutic drugs with immune checkpoint blockade anti-PD-1. In vivo data indicated that anti- PD-1 monotherapy didn’t significantly delay tumor growth nor prolong mice survival. However, when combined with chemotherapeutic drugs, especially gemcitabine, the tumor grew much slower, and mice got an obvious longer survival (Figure 4).
Figure 4: Anti-PD-1 treatment combining chemotherapeutic drug inhibited the tumor growth and extended the survival time of NSCLC xenograft mouse model. A: Tumor volume growth was inhibited in the NSCLC xenograft mouse model treated with carboplatin+anti-PD-1, paclitaxel + anti-PD-1 or gemcitabine+anti-PD-1 compared with anti-PD-1 treated group or the control group. B: The survival curve of NSCLC xenograft mouse model. The survival time of mouse treated with carboplatin+anti-PD-1, paclitaxel + anti-PD-1 or gemcitabine +anti-PD-1 was extended compared to the control group or anti- PD-1 treated group.
Gemcitabine synergizes with anti-PD-1 via enhancing anti-tumor immune mechanisms
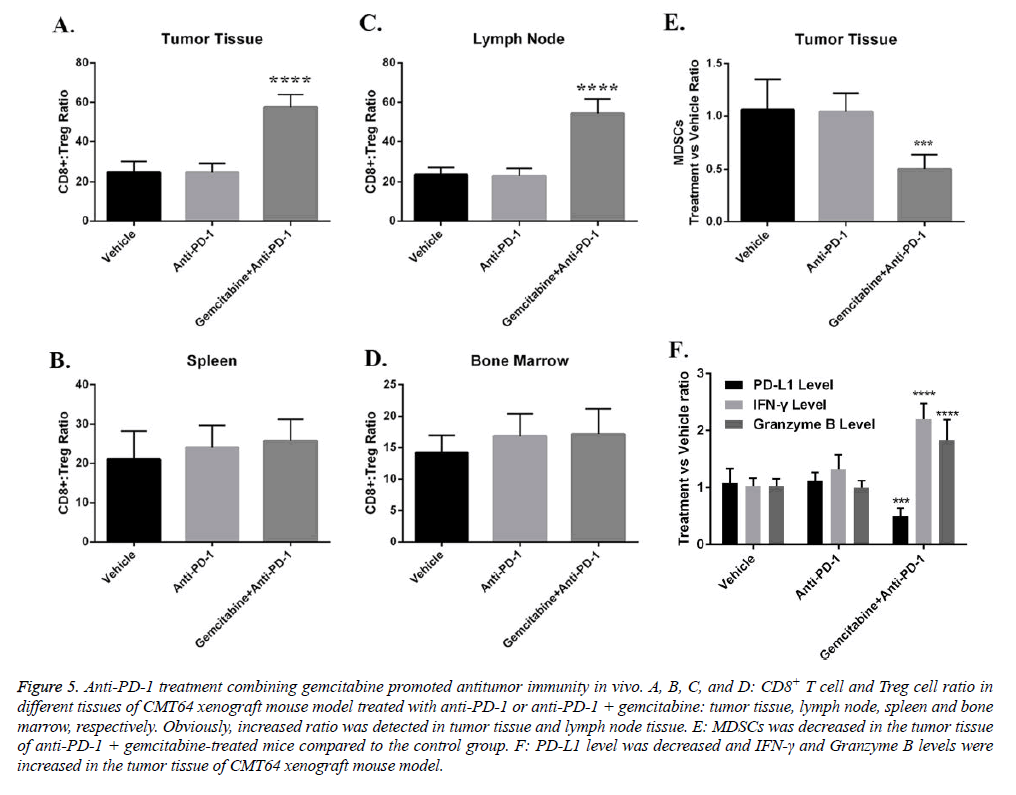
Mechanistically, we investigated the role of gemcitabine on immune inhibitory factors in anti-tumor immune process. We found that one of the primary the anti-tumor immunity inhibitory cell type; regulatory T cells were suppressed by gemcitabine. The ratio of CD8+ T cells versus Treg increased dramatically in tumor tissue and tumor draining lymph nodes (Figures 5A-5D). MDSC is another major cell type that inhibits T cell function. Our data indicated that administration of gemcitabine also depleted MDSCs in tumor tissue (Figure 5E). Cytokines that regulate T cell anti-tumor responses also changed by gemcitabine (Figure 5F). Gemcitabine treatment decreased PD-L1 but increased IFN-γ in tumor tissue (Figure 5F).
Figure 5: Anti-PD-1 treatment combining gemcitabine promoted antitumor immunity in vivo. A, B, C, and D: CD8+ T cell and Treg cell ratio in different tissues of CMT64 xenograft mouse model treated with anti-PD-1 or anti-PD-1 + gemcitabine: tumor tissue, lymph node, spleen and bone marrow, respectively. Obviously, increased ratio was detected in tumor tissue and lymph node tissue. E: MDSCs was decreased in the tumor tissue of anti-PD-1 + gemcitabine-treated mice compared to the control group. F: PD-L1 level was decreased and IFN-γ and Granzyme B levels were increased in the tumor tissue of CMT64 xenograft mouse model.
Discussion
Lung cancer is the top reason for male and second reason for female cancer-caused death worldwide [1,2]. Current treatments including surgery, radiotherapy, chemotherapy and targeted therapy prolonged survival of advanced NSCLC patients, but none of them showed curative effects [19]. Different from these traditional therapies which mainly targeted on malignant cells, immunotherapies eliminate tumor cells via motivating anti-tumor immune responses. Because the nature of immune therapies, they showed a long-time curative effect in advanced melanomas that are relative sensitive [17]. However, the proportion of immunotherapy sensitive solid tumor patients, such as NSCLC is small [20]. Therefore breaking intrinsic resistance is critical to improving immunotherapies in cancers [7]. Here, we showed that combining chemotherapeutic drugs, especially gemcitabine enhanced response to immune checkpoint blockades in NSCLC.
We started with proving the effects of commonly used chemotherapeutic drugs paclitaxel, carboplatin, and gemcitabine on NSCLC models. Then the immunogenicity inducing roles of these drugs were tested in KLN205 and CMT64 cell lines. HMGB1 releasing is a surrogate marker of immune cell death. Among all three treatments, gemcitabine treatment showed highest HMGB1 releasing, indicating the potential of combining gemcitabine with immunotherapies. Previous studies have shown that chemotherapy is a potent inducer of immune cell infiltration in tumor tissue [21]. Pre-treatment or during treatment, T cell infiltration is a critical foundation of immune checkpoint blockades [6,13]. Therefore, the T cell infiltration inducing roles of each drug was then determined. We found that although all drugs showed certain effects on enhancing T cell infiltration in tumor tissues, gemcitabine is strongest. Based on these data, we further hypothesized that using gemcitabine in combination with immune checkpoint blockades, such as anti-PD-1 will show significant synergistic effects in NSCLC models. Indeed, our preclinical data proved that when gemcitabine was combined, anti-tumor efficacy of anti-PD-1 treatment was enhanced dramatically.
To dissect the mechanisms by which gemcitabine sensitizes NSCLC xenograft tumors to anti-PD-1 treatment, we analyzed factors that regulate anti-tumor immunity. Treg cells were usually immune suppressive and dampened CD8+ T cells mediated anti-tumor immunity [22,23]. The ratio of CD8+ T cells versus Treg cells can represent the anti-tumor potency of the immune system. We found that in tumor tissue and tumor draining lymph nodes, the CD8+ T cell versus Treg ratio increased by gemcitabine and anti-PD-1 combinational treatment. These data were consistent with previous studies that indicated gemcitabine could inhibit Treg cells function [24,25]. MDSCs are another cell population that inhibits the anti-tumor activity of CD8+ T cells. It was shown that gemcitabine depleted MDSCs in tumors [26]. In line with these findings, our data also indicated that the number of MDSCs decreased after gemcitabine and anti-PD-1 treatment. Besides the changes in the cellular component, a T cell function boosting cytokine phenotype was seen after and anti-PD-1 treatment. The change of cytokines may be generated by depletion of inhibitory immune cells. Taken together, our data support that gemcitabine sensitizes NSCLC to anti-PD-1 therapy via dampening immune inhibitory factors in tumors.
Combinational therapies have been proposed as promising strategies to overcome the intrinsic resistance of immune checkpoint blockades in cancers [7]. In summary, we found that in NSCLC which was considered as insensitive to immune checkpoint blockades, combining chemotherapeutic drugs, such as gemcitabine will enhance response to anti-PD-1 treatment. In further steps, more preclinical and clinical studies are highly needed to evaluate the efficacy of chemotherapy and immune checkpoint blockades combination in NSCLC.
Acknowledgements
This work was supported by the internal fund from the Dongguan Third People’s Hospital, Dongguan, Guangdong, China, 523326
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: A Can J for Clincns. 2015; 65: 5-29.
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. In: Ahmad A, Gadgeel S, editors. Lung Cancer and Personalized Medicine: Curr Knowl and Therap 2016; 1-19.
- Fossella FV, DeVore R, Kerr RN. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000; 18: 2354-2362.
- Shepherd FA, Dancey J, Ramlau R. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000; 18: 2095-103.
- Zhou C, Wu YL, Chen G. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. The Lanc Oncol 12: 735-742.
- Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Sem in Oncol 2010; 37: 430-439.
- Sharma P, Allison James P. Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies with Curative Potential. Cell 161: 205-214.
- Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J National Can Inst 2007; 99: 1441-1454.
- Zhao X, He Y, Chen H. Autophagic tumor stroma: mechanisms and roles in tumor growth and progression. Int J of Can 2013; 132: 1-8.
- Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14: 1014-22.
- Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annual Review of Immunol 2001; 19: 565-594.
- Taube JM, Klein A, Brahmer JR. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Can Res 2014; 20: 5064-5074.
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. Journal clin oncol 2015; 33: 1974-1982.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012; 12: 252-264.
- Simeone E, Ascierto PA. Immunomodulating antibodies in the treatment of metastatic melanoma: the experience with anti-CTLA-4, anti-CD137, and anti-PD1. J Immune Toxicol 2012; 9: 241-247.
- Schadendorf D, Hodi FS, Robert C. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015; 33: 1889-1894.
- Prieto PA, Yang JC, Sherry RM. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Can Res 2012; 18: 2039-2047.
- Zamarin D, Postow MA. Immune checkpoint modulation: rational design of combination strategies. Pharmacology & therapeutics 2015; 150: 23-32.
- Ramnath N, Dilling TJ, Harris LJ. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e314S-40S.
- Garon EB, Rizvi NA, Hui R. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. New England J Med 2015; 372: 2018-28.
- Hong M, Puaux AL, Huang C. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer research 2011; 71: 6997-7009.
- Chen ML, Pittet MJ, Gorelik L. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005; 102: 419-24.
- Liyanage UK, Moore TT, Joo HG. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002; 169: 2756-2761.
- Shevchenko I, Karakhanova S, Soltek S. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int J Canc 2013; 133: 98-107.
- Rettig L, Seidenberg S, Parvanova I. Gemcitabine depletes regulatory T-cells in human and mice and enhances triggering of vaccine-specific cytotoxic T-cells. Int J Canc 2011; 129: 832-838.
- Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol 2009; 9: 900-909.