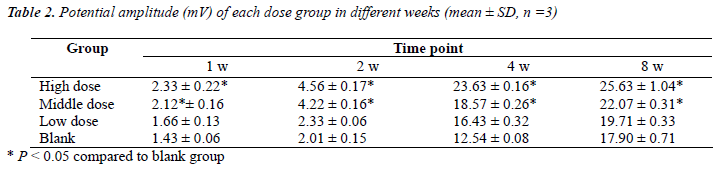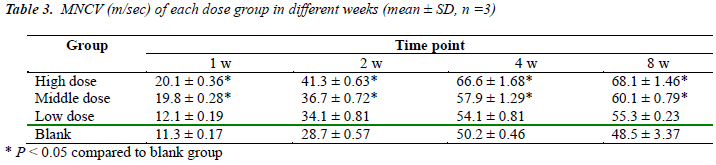ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2012) Volume 23, Issue 2
Immunosuppression of NF-?B by intragastric brazilein in motor neuron of spinal cord connected with injured sciatic nerve in mouse
1Department of Orthopedics, ChiFeng Municipal Hospital, Chifeng 024000, China.
2Department of Hand Surgery, China-Japan Union Hospital of Jilin University, Changchun 130033, China.
†The authors contributed equally to this work..
- *Corresponding Author:
- Lisen Li and Yuhong Man
Department of Hand Surgery
China-Japan Union Hospital
Xiantai Street 126#
Changchun 130033, China
Accepted Date: March 26 2012
Objective: The purpose of this study is to explore the immunosuppression of nuclear factorkappa B (NF-κB) by intragastric brazilein in spinal cord segments connected with injured sciatic nerve in mice. Methods: Healthy adult BALB/c mice underwent unilateral sciatic nerve interruption and anastomosis and then were treated with physiological saline (blank group), high dose, middle dose and low dose of brazilein in separate groups. The potential amplitude and motor nerve conduction velocity (MNCV) were measured. Myelin was assayed by immunohistochemical staining. NF-κB levels were detected by Western blotting and real-time PCR. Results: The potential amplitude and MNCV in the high and middle dose groups were significantly higher than in the low dose group and blank group. Myelin of the high dose and middle dose groups had more integrity. NF-κB was activated in spinal cord L4–6 connected with injured sciatic nerve. During the survival time of 12 h, 24 h, 3 d, 5 d and 1 w, NF-κB expression of the high dose group and the middle dose group were significantly lower versus the low dose and bland groups (P < 0.05). Conclusion: Brazilein can inhibit the immune activation of NF- κB in neurons in spinal cord L4–6 connected with injured sciatic nerve, which promotes nerve regeneration.
Keywords
Brazilein, Inhibition, Injury of sciatic nerve, NF-κB
Introduction
Immunosuppressant brazilein acts as the active constitu- ents of antibacterial Caesalpinia sappan [1], prevents the induction of immunological tolerance caused by high dose sheep red blood cells (SRBC) [2], resists the com- plement system and serves as an important immunosup- pressive component from Caesalpinia sappan L [3].
Injured peripheral nerves experience distal Wallerian degeneration and proximal retrograde degeneration. The inflammatory reaction plays a key role in these degenerations after injury, which not only affects the degeneration and regeneration of peripheral nerves, but also triggers pathological neuralgia. NF-κB (nuclear factor kappa B) as a ribonucleoprotein factor has been first reported [4] that can specifically bind to κB enhancer of immunoglobulin κ-chain gene. It is widely expressed in the nervous system and remains inactivation in the cytoplasm of arrest cells. NF-κB can be activated due to cell injury and then the activated NF-κB is transferred from the cytoplasm to the nuclei and binds to specific gene sequences that triggers the transcriptions of target gene Bcl-2 (B-cell lymphoma/ leukemia-2), inducible nitric oxide synthase (iNOS) and adhesive molecules [5,6] and regulates the immune and inflammatory reaction in patients. Thereupon, NF-κB plays a key role in the survival, plasticity, neural degeneration and neuropathic pain of injured neurons [7]. However, there is no report regarding the immunosuppression of NF-κB by brazilein administration in the spinal cord of the animals with sciatic nerve injury. In this paper, brazilein was dosed to sciatic nerve injury mouse model. The NF-κB expression in the spinal motor neurons was detected to assess the immunosuppression by intragastric brazilein in the growth process of sciatic nerve.
Methods
Reagents
Caesalpinia sappan ethanol extracts (containing 5% brazilein) were purchased from ChangYue Phytotech, Co., ltd. (Shaanxi, China) and brazilein was extracted from Caesalpinia sappan ethanol extracts as follows. Ethanol extract was concentrated at 50°C reduce pressure to sepa- rate water and ethanol and the ethanol portion was extracted with petroleum ether. Petroleum ether extract was concentrated under normal atmospheric pressure. The obtained powder was identified by HPLC (95% purity). Powder was dissolved in dimethylsulfoxide (DMSO) and subsequently mixed with physiological saline to form a concentration of 100 μg/ml. The final volume concentra- tion of DMSO in physiological saline was 0.05%. Brazil- ian and Caesalpinia sappan ethanol extracts were filtrated through 0.22 μm membrane for use.
Experimental animals and grouping
Total 460 healthy adult male BALB/c mice with weight of 25 ± 2 g were donated by Laboratory Animal Center of Fundamental Medical College of Jilin University and were raised at normal temperature with routine diet and free drink. They were randomized into blank group (treated with physiological saline), high dose, middle dose and low dose groups, respectively, 80 mice each group. The animal experimental protocols were approved by Laboratory Animal Ethnics Committee of Jilin University (No. JLU20080421009LA).
Animal modeling
All BALB/c mice were narcotized by intraperitoneal injection of 1% sodium thiopental with dose of 100 mg/(kg·weight). Mice were fixed in the prone position, 2 cm-longitudinal incisions were aseptically prepared on unilateral rear thigh and infrapiriformis sciatic nerves were dissected. Major sciatic nerves were carefully separated by blunt glass needles from surrounding tissues, sciatic nerves were completely interrupted in 0.5 cm below ischial tuberosity and microsurgically anastomosed by 11/0 microsutures with the aids of microscope with ×12 magnification, closing muscles and skins.
Dosing
Brazilein was dissolved in physiological saline and was intragastrically (ig) dosed to mice. Intragastric dose of mice was determined by equivalent conversion on the basis of clinical dose of brazilein [8], this dose value served as the middle dose and the high and low doses were determined by geometric progression. The final high, middle and low doses were 16, 8 and 4 g/kg/d, respectively. The physiological saline of equivalent volume served as control (blank group). Dosing was continuously until sacrifice.
Electroneurophysiological detection
Sciatic nerves of unilateral interruption were detected by a Keypoint® electromyograph & evoked potential apparatus (Medtronic A/C, Skovlunde, Denmark) 1 w, 2 w, 4 w and 8 w after dosing, respectively. Briefly, mice were narcotized as abovementioned and placed in 24ºC. After operation field disinfection, sciatic nerves were dissected.
Pin electrodes (M point) were placed into muscle belly of musculi soleus of mice for wave recording and grounding electrodes were placed to tails of mice. Single current stimulus (10 mA) was imposed to proximal anastomotic ischial tuberosity (P point) and distal sciatic nerve branch (D point) respectively by a parallel stimulating electrode with a fixed space of 2 mm between two tips. Distance between P point and D point were measured with a vernier caliper and the numerical value was input to obtain motor nerve conduction velocity (MNCV): MNCV = Distance between P point and D point/Differential value of potential latency. Functional recovery of sciatic nerves was determined by amplitude and MNCV.
Sampling of spinal cord
Ten mice of each group were narcotized by intraperitoneal injection of 1% sodium thiopental with a dose of 100 mg/kg in predetermined time points (12 h, 24 h, 3 d, 5 d, 7 d, 2 w, 4 w and 8 w after operation, respectively). Canalis vertebralis was exposed by rongeur via posterior vertebral column midline incision, the spinal cord segment L4–6 connected with injured sciatic nerves were intactly dissected, dissociated and removed and then these tissue samples were rapidly stored in liquid nitrogen for use in real time PCR and Western blotting.
Five mice from each group were fixed by supine position individually. Perfusion needle was placed to the aortic root by left ventricular cardiac apex. Rapid perfusion with 50-100 ml of physiological saline was performed to wash the blood. As the outflow from auricula dextra became limpid, perfusion was performed with 300-400 ml of fresh 4% paraformaldehyde in phosphate buffered saline. The perfusion was fast followed by slow and continuously lasted 30 min for tissue fixation. After the completion of the perfusion, canalis vertebralis was exposed by rongeur, the spinal cord segment L4–6 connected with injured sciatic nerves were intactly dissected, dissociated and removed and then these tissue samples were fixed 72 h in 10% neutral formaldehyde, were dehydrated by gradient ethanol and were embedded by paraffin. These embedded specimens were stored until the immunohistochemical staining was performed.
In 8w and after electroneurophysiological detection, sciatic nerve cords between from anastomosis (including anastomotic stoma) to distal 0.6-0.7 cm were cut off, then were fixed 72 h in 10% neutral formaldehyde, were dehydrated by gradient ethanol and were embedded by paraffin. These embedded specimens were stored until the staining with luxol fast blue (LFB) was performed.
Western blotting
Tissue samples stored in liquid nitrogen were taken out quickly and grinded in a mortar. Cells were lysed in icecold radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, Nanjing, China) for 15 min. Proteins were separated on an 10% SDS-PAGE gels and electroblotted onto a polyvinylidine fluoride (PDVF) film. PDVF film was incubated in rabbit anti-mouse NF-κB antibodies (diluted 1,000 times with PBS containing 1% BSA; Beyotime, Nanjing, China) overnight in 4°C and rinsed 4 times by 0.01 mol/L PBS, 5 min each time. Color was developed by using Western Blotting DAB Testing Kit (Beyotime, Nanjing, China), X-ray film exposure were performed, then scanning and analyzing.
Real-time PCR
Primers of NF-κB and GAPDH were designed by Beacon Designer 7 software (Table 1) and were identified for specificity via the BLAST retrieval. Total RNAs were extracted from tissue samples using TRIzol and cDNA was cloned by reverse transcriptase using total RNA templates. NF-κB genes were cloned by real-time PCR using cDNA templates and a pair of GAPDH primers served as inner control in each reaction system. The reaction condition: 95°C, 30s; 58°C, 60s; 72°C, 60s; 40 cycle reactions.
Immunohistochemical staining of NF-κB/p65
NF-κB/p65 protein was detected by immunohistochemical SABC method. Specimens were sectioned into pieces of 2 μm, dewaxed and hydrated. Antigen repair was performed. Slices were exposed to 50 μL of dropwise rabbit anti-mouse NF-κB/p65 antibody (1: 200) in 4ºC overnight. Slices were then exposed to biotinylated goat anti-rabbit IgG 20 min in room temperature and exposed to SABC 20 min in room temperature. DAB was used for color development. Redyeing with haematoxylin, dehydration and vifrification was performed successively. Slices were sealed for microscopic observation.
Histological staining
After embedded specimens were sectioned into pieces of 2 μm and dewaxed, slices were immersed 12 h in luxol fast blue (LFB) solution at 60ºC, were immersed 5 min in 95% ethanol, were incubated 15 sec in 0.05% lithium carbonate solution and were washed by 70% ethanol and distilled water respectively. After dehydration and vitrification, slices were sealed for microscopic observation.
Statistical analysis
SPSS 10.0 software was used for statistical analysis and ttest was conducted for comparison. Data were stated as mean ± SD (standard deviation), P < 0.05 indicated statistically significant difference.
Results
Electroneurophysiological detection
Results of potential amplitude and MNCV were shown in Table 2 and Table 3, respectively. The amplitude and MNCV of both high and middle dose groups in 1 w, 2 w, 4 w and 8 w respectively were statistically significantly higher than the blank group (P < 0.05), but there was not statistically significant difference between high dose and middle dose groups or between low dose and blank groups (P > 0.05). These results indicated that use of brazilein in Balb/c mouse with injured sciatic nerve can accelerate the functional recovery and regeneration of nerves.
Western blot analysis
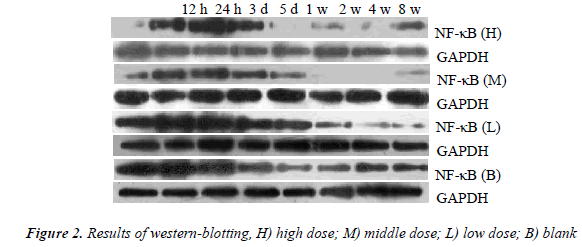
Figure 2 shows the results of western blot. Grayscale analysis demonstrated the level of NF-κB protein in each group increased after sciatic nerve injury, then NF-κB in the high dose group decreased to a normal level in 1 w, NF-κB in the middle and low dose groups decreased to the normal levels in 2 w and in 4 w respectively, but NF- κB in the blank group remained a high level in 8 w. The levels of NF-κB between time points of post-operative 12 h, 24 h, 3 d, 5 d and 1 w in each experiment group had statistically significant differences (P < 0.05). NF-κB expression in the high and middle dose groups was significantly inhibited compared to the blank group (P < 0.01).
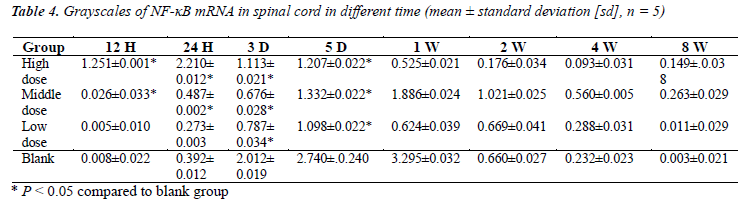
Real-time PCR analysis
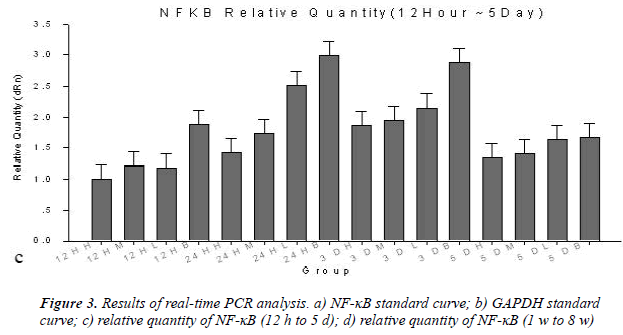
NF-κB mRNA is little detected in normal sciatic nerves in mice. Grayscales of NF-κB mRNA in spinal cord and results of real-time PCR analysis were shown in Table 4 and Figure 3, respectively. After sciatic nerve injury, NF- κB mRNA content in spinal cord segments connected with inured sciatic nerve was increased in a short term and the increment in both high and middle dose groups was significantly less than those in both low dose group and blank group (P < 0.05). After time point of 2 w, NF- κB mRNA content had no statistically significantly difference between groups. These results demonstrated that the high and middle dose of brazilein obviously suppressed the expression of NF-κB.
Immunohistochemical staining
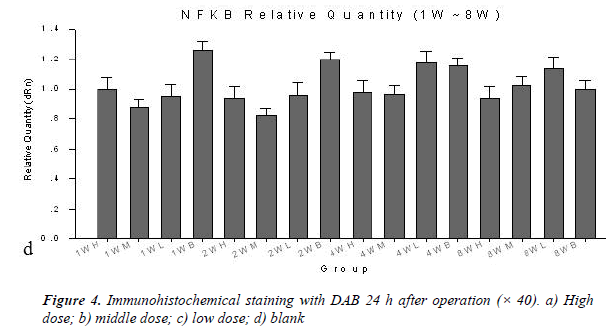
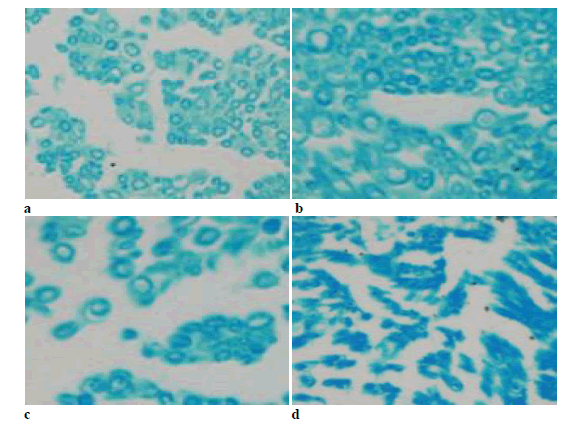
The immune positives were mainly present in the cytoplasm of motor neurons. The staining results showed that there was significant difference between groups 24 h after operation that the high dose and middle dose groups had lower positives than the low dose and blank groups (Figure 4).
Histological staining
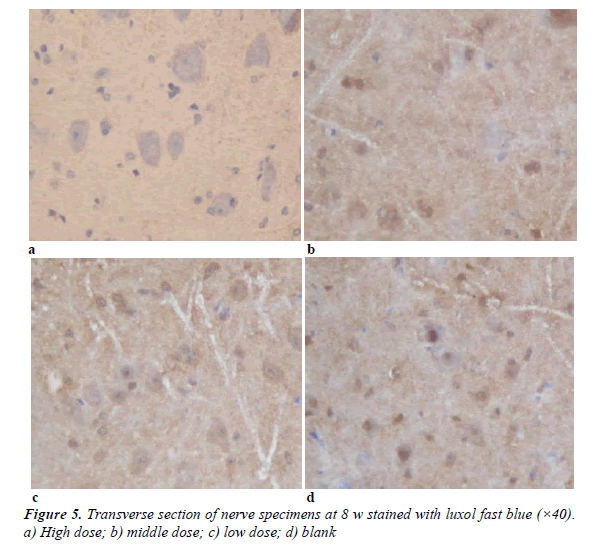
Nerve specimens in 8w stained with LFB were shown in Figure 5. Myelin was dyed in blue but axons with a white background were not dyed. Myelin of the high dose and middle dose groups presented regular shapes, uniform thickness, sharp outlines and little fibroplastic proliferation around myelin. Myelin of the low dose group presented irregular shapes and thickness, moderately sharp outlines and the interfascicular proliferation of fibrous connective tissues. Myelin of the blank group presented the worst shape and the obviously proliferation of fibrous connective tissues.
Discussion
Brazilein, one of main components of Caesalpinia sappan is the oxide of brazilin that is vulnerable to air and light and can be extracted in large scale in organic solvent [9]. Brazilin also has many biological effects to resist bacteria as the active constituents Caesalpinia sappan [1]) and to prevent the induction of immunological tolerance caused by high dose sheep red blood cells (SRBC) [2].
In vitro and in vivo trials [3] showed the immunosuppressive properties of brazilein. By long-term expose to brazilein, weight coefficients of the thymus and spleen in mice are reduced, T-lymphocyte proliferation induced by ConA is inhibited, and B lymphocyte proliferation induced by LPS is also suppressed [1]. Flow analysis results show that the immunosuppression may associate with lymphocyte apoptosis induced by brazilein. However, other biological effects of brazilein have seldom been reported.
NF-κB as a key nuclear transcription factor widely exists in eukaryotes and is present in the cytoplasm by inactive homologous or heterologous dimers to form complexes with its inhibitory protein IκBs [4,10]. By sorts of internal and external stimuli (some cytokines, growth factors, immunity receptors, ischemia and anoxia, and injury), NF- κB dissociates from IκBs and is quickly transferred into the nucleus and binds to target gene promoter or enhancer κB motif to modulate the synthesis of mRNA of target genes, by which NF-κB participates in various biological processes such as immune response, inflammation, apoptosis and cell proliferation [5-7,11,12]. NF-κB has multiple regulatory action on gene transcription that binds to immunoglobulin gene κ-light chain enhancer κB specific sequences [6,13] and participates in the inflammatory nerve lesions, the pathologic nerve pain and the function change after nerve injury [14], inhibits axonal regeneration and promotes neuronal apoptosis [6,15].
Brambilla et al.[16] found the immunosuppression of NF- κB in astrocytes can alleviate inflammatory response after spinal cord injury and promote the functional recovery of spinal cord. It is the prerequisite to protect neuronal soma and avoid irreversible degeneration for successful regeneration after nerve injury. It will help nerve regeneration to promote injured neuron survival and inhibit neuron degeneration after nerve injury.
In this study, the results based on Western blot, real time PCR and immunohistochemical staining showed NF-κB was activated owing to sciatic nerve injury and then was expressed in a large amount in spinal cord segment connected with injured sciatic nerve; after brazilein was intragastrically dosed, the expression of NF-κB in the high dose and middle dose groups was significantly lower than that in the low dose and blank group. In addition, the potential amplitude and MNCV are positively associated with the integrities of myelin. The results showed after brazilein was intragastrically dosed, the myelin of the high dose and middle dose groups had more integrity and the potential amplitude and MNCV in the high and middle dose groups were significantly higher than in the low dose group and blank group.
In conclusion, use of brazilein after sciatic nerve injury can suppress the immune activation of NF-κB in cornu anterius medullae spinalis and reduce apoptosis, which promotes the nerve regeneration.
Acknowledgements
This study was funded by Jilin Science and Technology Bureau Project No. 20080934 and Jilin University Doctor Foundation No. 20030183014.
References
- Xu HX, Lee, SF. The antibacterial principle of Caesal- pina sappan. Phytother Res 2004; 18: 647-651.
- Mok MS, Jeon SD, Yang KM, et al. Effects of brazilin on induction of immunological tolerance by sheep red blood cells in C57BL/6 female mice. Arch Pharm Res 1998; 21: 769-773.
- Ye M, Xie WD. Brazilein, an important immunosupp- ressive component from Caesalpinia sappan L. Int J Immunopharmacol 2006; 6: 426– 432
- Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986; 46 (5): 705-716.
- Boersma MC, Dresselhaus EC, De Biase LM, Mihalas AB, Bergles DE, Meffert MK. A Requirement for Nu- clear Factor-{kappa}B in Developmental and Plasti- city-Associated Synaptogenesis. J Neurosci 2011; 31 (14): 5414-5425.
- Perkins ND. Integrating cell-signaling pathways with NF-kB and IKK function. Nature Reviews Mol Cell Biol 2007; 8: 40-62
- Meffert MK, Chang JM, Wiltgen BJ, et al. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci 2003; 6 (10): 1072-1078.
- Huang J, Huang X, Chen Z, et al. Dose conversion among different animals and healthy volunteers in pharmacological study. Chin J Clin Pharmacol Ther (Chin) 2004; 9 (9): 1069-1072
- de Oliveira Luiz FC, Edwards-Howell GM, Velozo- Eudes S, et al. Vibrational spectroscopic study of brazi- lin and brazilein, the main constituents of brazilwood from Brazil. Vibr Spectro 2002; 28: 243–249.
- Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene 2006; 25 (51): 6680-6684
- Brasier AR. The NF-kappa B regulatory network. Car- diovase Toxicol 2006; 6(2): 111-130
- Perkins ND. Integrating cell-signalling pathways with NF-kappa B and IKK function. Nat Rev Mol Cell Biol 2007; 8 (1): 49-62
- Wang CY, Mayo MW, Korneluk RG, et al. NF-κB an- tiapoptosis: induction of TRAF1 and TRA F2 and c- IAP1 and c-IAP2 to suppress caspases-8 activation. Science 1998; 281 (5383): 1680 -1683
- Sanchez-Ponce D, Tapia M, Munoz A, et al. New role of IKKα/βphosphorylated IkBαin axon outgrowth and axon initial segment development. Mol Cell Neurosci 2008; 37 (4): 832-844.
- Svaren J, Meijer D. The molecular machinery of mye- lin gene transcription in Schwann cells. Neurosci Lett 2008; 56(4):1541-1554.
- Brambilla R, Bracchi-Ricard V, Hu WH, et al. Inhi- bition of astroglial nuclear factor kappaB reduces in- flammation and improves functional recovery after spi- nal cord injury. J Exp Med 2005; 202 (1):145-156.