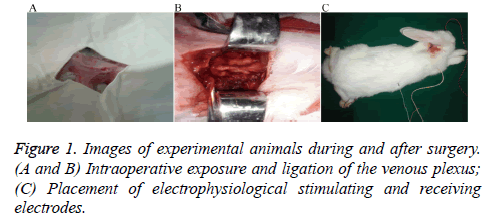
ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 16
Impact of posterior atlantoaxial joint venous plexus ligation on spinal cord function: evaluation according to imaging, electrophysiology, and ethology
Huawei Wei, Zonghua Qi, Xin Liang, Jing Fang, Lianchong Fu, Jiankun Yang, Deling Yang, Xu Zhang, Shilong Yuan, Feng Gao, Siyuan Li and Yongming Xi*
Department of Spinal Surgery, the Affiliated Hospital of Qingdao University, Qingdao, PR China
- *Corresponding Author:
- Yongming Xi
Department of Spinal Surgery
The Affiliated Hospital of Qingdao University
Qingdao, PR China
Accepted date: July 21, 2017
The aim of this study was to assess the impact of bilateral ligation and amputation of the posterior atlantoaxial joint venous plexus on spinal cord function. Healthy New Zealand white rabbits were selected and examined using somatosensory evoked potentials, motor evoked potentials, and Magnetic Resonance Imaging (MRI) MAR GE Sigma 1.5-T MR system (GE, Milwaukee, USA) (Slice thickness: 3 mm, T1-Weighted Image (T1WI): TR/TE=400/15 ms, T2-Weighted Image (T2WI): TR/TE=3500/102 ms). Electrophysiology (MedCom Technology Co., Ltd. Zhuhai, China) was performed at specific time points (2, 6, and 12 h) after the bilateral posterior atlantoaxial venous plexus was ligated and amputated. MRI was also performed at specific time points (immediately after surgery, and at 8 and 48 h after surgery). The mobility of the hind limbs was evaluated before surgery and at 1, 3, and 7 d after surgery. No hind limb motor dysfunction was found at different time points. Tarlov scores were all grade 5. The spinal cord area and relative T1WI and T2WI intensities showed no significant differences at specific time points before and after surgery. Electrophysiological signals, including amplitude and latency, showed no significant differences at specific time points before and after surgery. Ligation and amputation of the posterior atlantoaxial joint venous plexus does not affect spinal cord blood supply or cord function.
Keywords
Atlantoaxial, Venous plexus, Spinal cord function, Electrophysiology, MRI
Introduction
A variety of mechanisms can destroy the integrity and continuity of the axial odontoid process or transverse atlantal ligament, thus leading to atlantoaxial joint dislocation [1-4]. According to severity, dislocation can be classified as reducible or irreducible. In the past few decades, posterior internal fixation for irreducible atlantoaxial dislocation has been performed using the Gallie method and Brooks’s method [5-7]. These two methods use steel wire for internal fixation. However, the wire can damage the spinal cord, and can easily break. In 1985, Magerl proposed transatlantoaxial screw fixation, which is not dependent on the posterior arch, and therefore has better stability and postoperative fusion rates [8,9]. However, this method requires complete reduction of the atlantoaxial joint, with a high risk of vessel injury in patients with a high-riding vertebral artery. Goel et al. proposed reduction fixation after posterior transarticular process release, but this method requires transection or ligation of the posterior atlantoaxial joint venous plexus, and can easily lead to significant intraoperative blood loss [10,11]. Most patients with atlantoaxial dislocation exhibit significant expansion of the posterior atlantoaxial joint venous plexus, which even forms a large venous sinus; whether this expanded and disrupted venous plexus can lead to local blood reflux in the spinal cord, thereby affecting spinal cord function, has not been reported. In this study, we assessed the effect of bilateral ligation and amputation of the posterior atlantoaxial joint venous plexus according to imaging, Electrophysiology (ECP), and ethology.
Materials and Methods
Animals
Fifteen New Zealand white rabbits (Qingdao Institute for Food and Drug Control, China; aged 5-6 months, weighing 2.6-3.0 kg, female/male-unrestricted) were all fed for 2 weeks under the same experimental conditions. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Qingdao University.
Treatment of the animals
All rabbits received intramuscular injections of 400,000 U of penicillin, 30 min before surgery, and were anesthetized by intramuscular injection of 10 mg/kg ketamine hydrochloride. Room temperature was held constant at 24-26°C. After successful general anesthesia induction, 20-25% of the initial dose was supplemented according to the need for satisfactory anesthetic effect [12,13]. Each rabbit was then placed in the prone position, with limbs fixed on the operating table. Occipitocervical fur was removed, skin was disinfected using povidone iodine and draped with towels, and longitudinally incised from the occipital protuberance to the 4th spinous process (about 7 cm in length). Following local anesthesia with 2% lidocaine, tissues were incised and separated by layers. The skin, subcutaneous tissue, and paraspinous process muscles and ligaments were pulled aside using an automatic retractor to expose the venous plexus for bilateral amputation with cautery (Figure 1). After surgery, the tissues were disinfected and the muscles and skin were closed in layers. Routine postoperative administration of penicillin 400,000 U × 3 d was performed, and the recovering rabbit was kept at constant room temperature and fed under standard conditions.
Observation indexes
Preoperative somatosensory evoked potentials and motor evoked potentials, as well as Magnetic Resonance Imaging (MRI), were performed. (1) Spinal cord signal intensity: T1 weighted imaging (T1WI) and T2 weighted imaging (T2WI) signal intensities were measured from clear images obtained in the sagittal plane from the upper atlantal edge to the lower axial edge. Relative Signal Intensity (SI) within the spinal cord was assessed as follows: SI (r)=(Ss-Sn)/Sn13, where Ss was the signal intensity within the spinal cord and Sn was the background noise signal intensity within the same view field. (2) Spinal cord area: Images were located and measured from the upper atlantal edge to the lower axial edge, and the spinal cord area at this level was calculated; ECP was performed 2, 6, and 12 h after surgery. MRI was performed immediately at 8 and 48 h after surgery. Meanwhile, the rabbits were monitored for movement during recovery, and the hind limbs were evaluated 1, 3, and 7 d after surgery using the Tarlov scale.
Grade 5: Can walk normally.
Grade 4: Can walk with only mild difficulty.
Grade 3: Hind limbs can support body weight for 1-2 steps.
Grade 2: Hind limbs cannot bear weight with moderate or rapid activity.
Grade 1: Hind limbs can move, but cannot bear weight.
Grade 0: Hind limbs cannot move or bear weight.
Statistical analysis
All data were expressed as ͞x ± s, and SPSS 16.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. The spinal cord area and relative signal intensity were measured at different time points before and after surgery. The ECP signals, including amplitude and latency data, were analyzed using the least significant difference t-test, with P<0.01 indicating statistical significance.
Results
Evaluation of imaging signals
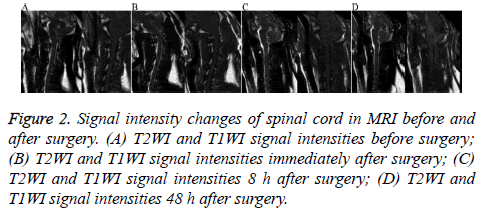
There was no significant difference in the spinal cord area or T1WI and T2WI relative signal intensity between the two groups at different time points before and after surgery (P=0.12, P>0.05, Table 1 and Figure 2).
Figure 2: Signal intensity changes of spinal cord in MRI before and after surgery. (A) T2WI and T1WI signal intensities before surgery; (B) T2WI and T1WI signal intensities immediately after surgery; (C) T2WI and T1WI signal intensities 8 h after surgery; (D) T2WI and T1WI signal intensities 48 h after surgery.
| Index | Before surgery | Immediately after surgery | 8 h after surgery | 48 h after surgery |
|---|---|---|---|---|
| Spinal cord area | 44.2 ± 3.1 | 44.3 ± 3.0 | 44.3 ± 3.2 | 44.2 ± 3.2 |
| T1WI relative signal intensity | 25.2 ± 2.8 | 25.7 ± 2.7 | 25.5 ± 2.6 | 25.4 ± 2.5 |
| T2WI relative signal intensity | 41.8 ± 3.2 | 41.6 ± 3.1 | 41.7 ± 3.0 | 41.5 ± 3.1 |
Table 1. Spinal cord area, T1WI, and MI relative signal intensities at different time points before and after surgery.
ECP
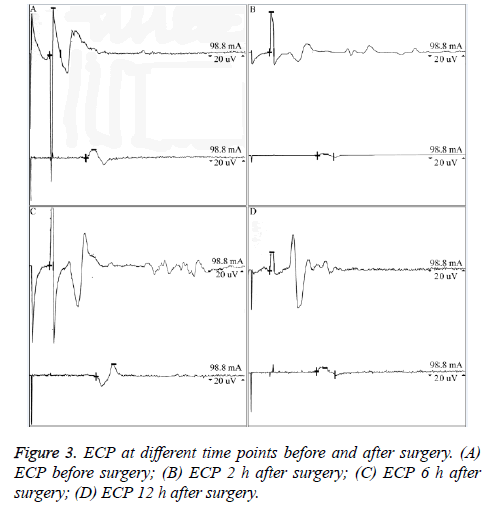
There was no significant difference in ECP signals, including amplitude and latency results, at different time points before and after surgery (P=0.09, P>0.05, Table 2 and Figure 3).
| ECP Amp (mv) | ECP Lat (ms) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-op | Post-op 2 h | Post-op 6 h | Post-op 12 h | Pre-op | Post-op 2 h | Post-op 6 h | Post-op 12 h | |
| Left wrist | 0.083 ± 0.02 | 0.399 ± 0.04 | 0.246 ± 0.06 | 0.032 ± 0.02 | 4.8 ± 0.5 | 4.6 ± 0.4 | 4.9 ± 0.7 | 4.7 ± 0.3 |
| Right wrist | 0.079 ± 0.02 | 1.498 ± 0.03 | 0.075 ± 0 .04 | 0.054 ± 0.03 | 4.4 ± 0.2 | 4.7 ± 0.3 | 4.8 ± 0.6 | 4.8 ± 0.4 |
| Left elbow | 0.014 ± 0.02 | 0.012 ± 0.13 | 0.022 ± 0.22 | 0.066 ± 0.32 | 12.9 ± 0.3 | 15.6 ± 0.2 | 15.4 ± 0.3 | 15.6 ± 0.4 |
| Left elbow | 0.022 ± 0.11 | 0.037 ± 0.22 | 0.011 ± 0.22 | 0.013 ± 0.14 | 12.8 ± 0.6 | 13.7 ± 0.4 | 15.3 ± 0.3 | 15.6 ± 0 .2 |
Table 2. Electrophysiological signals at different time points before and after surgery (amplitude, latency period).
Evaluation of motor function
There was no motor dysfunction in the bilateral hind limbs at different time points before and after surgery, and all rabbits had a grade 5 Tarlov score.
Discussion
There are a variety of treatment methods for irreducible atlantoaxial dislocation, including anterior, posterior, or anteroposterior joint approaches [14,15]. Because compression on the medullary spinal cord is mainly from the front, an anterior transoral or transnasal odontoid resection can achieve decompression [16,17]. Ai et al. applied the transoral approach to complete an anterior release as well as reduction and decompression assisted by plates [18-20]. In anterior dislocation of the atlas, the anterior longitudinal ligament, intrinsic cervical muscles, and contracted lateral mass joint capsule can affect surgical reduction. Cutting of all ligaments, muscles, and the capsule, aided by heavy cervical traction and instrumental probing, can assist in anatomical reduction of the atlantoaxial joint.
Wang et al. performed transoropharyngeal release, followed by posterior pedicle screw fixation [21]. However, transoral posterior pharyngeal surgery is associated with more complications, making perioperative management difficult [22]. Posterior surgeries for atlantoaxial dislocation include the Gallie method, the Brooks method, and the Magerl method [5,6,8]. The Gallie method and the Brooks method use steel wire for fixation, with obvious disadvantages. The wire fixation technique is difficult, and can prolong the operative time. As the spinal cord is easily damaged by the steel wire, these methods are rarely used alone. Goel et al. proposed reduction fixation after posterior transarticular process release, and achieved initial positive clinical results [10,11]. The advantage of this approach is that it can directly achieve reduction and fusion through surgical release, while requiring no anterior or posterior joint surgery. This method is especially suitable for patients with various types of arch deficiency. However, this method also requires transection of the posterior atlantoaxial joint venous plexus, thus increasing the possibility of significant intraoperative bleeding. Furthermore, whether complete bilateral transection can affect local circulation or even spinal cord function is still unclear.
The venous plexus lateral to the posterior atlantoaxial joint lies in the rear part of the joint as well as between the upper edge of the axial isthmus and the lower edge of the posterior atlantal arch, and comprises a rich network of prominent, bead-like vessels. The valveless venous plexus has thin walls, and haemostasis can be difficult after an injury. Batson described the vertebral venous plexus as composed of the plexus lateral to the posterior atlantoaxial articular dura mater and the extraspinal canal venous plexus [23]. This network can buffer the intracalvarial venous drainage caused by body flexion and changes in thoracic abdominal pressure, and can adjust intracranial pressure by transmitting respiratory effects and cardiovascular pressure changes, thus balancing the pressure in the venous system.
In this study, we examined the impact of ligating the posterior atlantoaxial venous plexus on spinal cord function in rabbits. The results revealed no significant difference in the spinal cord area or T1WI and T2WI relative signal intensities at different time points before and after surgery. There was also no significant difference in ECP signals, including amplitude and latency, in the experimental animals at different time points before and after surgery. In addition, there was no hind limb motor dysfunction at different time points before and after surgery, with all Tarlov scores graded 5. The Tarlov score, MRI signal area, T1WI relative signal intensity, and T2WI relative signal intensity in this study were correlated with each other. The results of this study showed that ligating the venous plexus will not affect the blood supply between the upper atlantal edge and the lower spinal cord edge, and will not cause spinal cord injury.
There are some limitations in this study. The sample size was small and the observation period was relatively short. Therefore, a long-term, large-scale observational study is required.
Conclusions
By calculating and analyzing the spinal cord area and the T1WI and T2WI relative signal intensities in the sagittal plane before and after ligating the posterior atlantoaxial joint venous plexus, as well as monitoring ECP signals (including the amplitude and latency), we can conclude that ligating the venous plexus does not affect the blood supply from the upper atlantal edge to the lower axial edge, and does not cause spinal cord injury.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Greenberg AD. Atlanto-axial dislocations. Brain 1968; 91: 655-684.
- Macovei LA, Rezuş E. Cervical spine lesions in rheumatoid arthritis patients. Rev Med Chir Soc Med Nat Iasi 2016; 120: 70-76.
- Wadia NH. Myelopathy complicating congenital atlanto-axial dislocation (A study of 28 cases). Natl Med J India 2016; 29: 38-56.
- He DW, Huang WJ, Sheng XY, Wu LJ, Fan SW. Atlantoaxial joint interlocking following type II odontoid fracture associated with posterolateral atlantoaxial dislocation: A case report and review of published reports. Orthop Surg 2016; 8: 405-410.
- Gallie WE. Fractures and dislocations of cervical spine. Am J Surg 1939; 46: 495-499.
- Brooks AL, Jenkins EB. Atlanto-axial arthrodesis by the wedge compression method. J Bone Joint Surg Am 1978; 60: 279-284.
- McCarron RF, Robertson WW. Brooks fusion for atlantoaxial instability in rheumatoid arthritis. South Med J 1988; 81: 474-476.
- Hanson PB, Montesano PX, Sharkey NA, Rauschning W. Anatomic and biomechanical assessment of transarticular screw fixation for atlantoaxial instability. Spine 1991; 16: 1141-1145.
- Stein G, Schiffer G, Bredow J, Meyer C. 3D Fluoroscopy-navigated magerl fusion of the 1st and 2nd cervical vertebra. Z Orthop Unfall 2016; 154: 636-637.
- Goel A, Desai KI, Muzumdar DP. Atlantoaxial fixation using, plate and screw method: A report of 160 treated patients. Neurosurg 2002; 51: 1351-1357.
- Goel A, Laheri V. Plate and screw fixation for atlanto-axial subluxation. Acta Neurochir 1994; 129: 47-53.
- Xi Y, Kong J, Liu Y, Wang Z, Ren S, Diao Z, Hu Y. Minimally invasive induction of an early lumbar disc degeneration model in rhesus monkeys. Spine 2013; 38: 579-586.
- Kim TH, Ha Y, Shin JJ, Cho YE, Lee JH, Cho WH. Signal intensity ratio on magnetic resonance imaging as a prognostic factor in patients with cervical compressive myelopathy. Med 2016; 95: 4649.
- Wang S, Wang C, Yan M, Zhou H, Dang G. Novel surgical classification and treatment strategy for atlantoaxial dislocations. Spine 2013; 38: 1348-1356.
- Xi Y, Zhang X, Yuan S, Cao J, Wang D, Ma J, Ren X, Hu Y. Occipitocervical fusion combining intra-operative skull traction with posterior instrumented reduction for the treatment of basilar invagination with atlantoaxial dislocation. Int J Clin Exp Med 2016; 9: 9101-9107.
- Wolinsky JP, Sciubba DM, Suk I, Gokaslan ZL. Endoscopic image-guided odontoidectomy for decompression of basilar invagination via a standard anterior cervical approach. J Neurosurg Spine 2007; 6: 184-191.
- Dasenbrock HH, Clarke MJ, BydonA, Sciubba DM, Witham TF, Gokaslan ZL, Wolinsky JP. Endoscopic image-guided transcervical odontoidectomy: Outcomes of 15 patients with basilar invagination. Neurosurg 2012; 70: 351-360.
- Ai FZ, Yin QS, Xu DC, Xia H, Wu ZH, Mai XH. Transoral atlantoaxial reduction plate internal fixation with transoral transpedicular or articular mass screw of c2 for the treatment of irreducible atlantoaxial dislocation: two case reports. Spine 2011; 36: 556-562.
- Yin Q, Ai F, Zhang K, Chang Y, Xia H, Wu Z, Quan R, Mai X, Liu J. Irreducible anterior atlantoaxial dislocation: one-stage treatment with a transoral atlantoaxial reduction plate fixation and fusion. Spine 2005; 30: 375-381.
- Zheng Y, Wu ZH, Yin YH, Yin QS, Zhang K. Treatment of irreducible atlantoaxial dislocation using the transoral atlantoaxial pedicle screw technique: A report of 10 cases. Orthopade 2016; 45: 174-178.
- Wang C, Yan M, Zhou HT, Dang GT. Open reduction of irreducible atlantoaxial dislocation by transoral anterior atlantoaxial release and posterior internal fixation. Spine 2006; 31: 306-313.
- Yin QS, Xia H, Wu ZH, Ma X, Ai F, Zhang K, Wang J, Zhang T, Bai Z, Wang Z. Surgical site infections following the transoral approach: A review of 172 consecutive cases. Clin Spine Surg 2016; 29: 502-508.
- Batson OV. The function of the vertebral veins and their role in the spread of metastases. Arch Surg 1940; 112: 138-149.