ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2018) Volume 29, Issue 10
Improvement of purification methods for F(ab')2 fraction of equine hyperimmune plasma against scorpion venom
1Department of Chemistry, Yazd Branch, Islamic Azad University, Yazd, Iran
2Razi Serum and Vaccine Research Institute, Ahvaz Branch, Agricultural Research, Education and Extension Organization (AREEO), Tehran, Iran
- *Corresponding Author:
- Razieh Mohebat
Department of Chemistry
Yazd Branch
Islamic Azad University, Yazd, Iran
Accepted date: July 07, 2017
DOI: 10.4066/biomedicalresearch.29-17-986
Visit for more related articles at Biomedical ResearchAntivenoms used for treatment of envenomation caused by like snakes and scorpions, are mostly produced in equine (horses) or ovine (sheep and goat). In Iran, the production of polyspecific scorpion antivenom has been started since 70 y ago and purified with ammonium sulfate fractionation after pepsin digestion of Fc fragment from IgG. Since this conventional method is time consuming, expensive and the product has lower purity. In this study we used either caprilic acid (Octatonic acid) alone or with combination with ammonium sulfate for separation and removal of impurities and precipitation of specific F(ab´)2 against scorpion venom. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) showed that purification with caprilic acid at a final concentration of 3.5% is optimal to obtain immunoglobulins essentially free of albumin. The second method of purification includes combination of 1.5% (v/v) caprilic acid with 20% saturation with ammonium sulfate. Both procedures are performed after peptic digestion of IgG (removal of Fc fragment) at 37°C for 4 h at pH=3.5. After peptic digestion pH is raised to 4.8 with 1.5 molar sodium hydroxide to stop enzyme digestion then in the first method 3.5-5% caprilic acid was added with strong agitation for one hour the impurities were removed with filter paper and the supernatant was dialyzed against CIS buffer. In the second method after adjusting pH=4.8 ammonium sulfate was added to 20% saturation then 1.5% caprilic acid was added with strong agitation for one hour then slurry is passed through filter paper the precipitated impurities discarded and supernatant was dialyzed.
Keywords
Caprilic acid (CA), Ammonium sulfate (AS), F(ab´)2, Fc fragment, Antivenom purification.
Introduction
Scorpion envenomation is considered as a problem in public health system of many countries of world. There are over 1.2 billion people living in areas the world where there is the possibility of scorpion envenomation, 1.2 million individuals are bitten by scorpion annually, that about 0.27% of them die from the bite [1,2]. The scorpion poison causes dermal ulcers and very severe inflammation at the site of bite. Also, severe complications are observed such as haemolysis and also articular problems in affected people [3]. Serum therapy is the only treatment way of scorpion-bitten people. Initially, the researchers had used serum of secured animals in order to treatment of affected people, although this method had a significant effect on treatment of patients, but it had many side effects [4,5]. In order to reducing these effects, the researchers applied antibody purification method. Previous studies have shown that the F(ab´)2 part of polyclonal antibody harvested from immune animals, has the most significant effect in treatment of these patients and on the other hand, it shows lower side effects compared to the total serum [6-8].
Therefore, the application of methods in which the F(ab´)2 part of antibody can be purified, has a significant importance. classically, in order to providing and concentrating of F(ab´)2 part of antibody, the method of sedimentation with Ammonium Sulfate (AS) with/without using of enzymatic or thermal digestion are used [9]. But this is a very costly and expensive method and on the other hand leads to decrease of antibody efficacy to levels lower than 50%, therefore, attempt for discovering alternative methods are of importance, methods such as acid, ion-exchange chromatography and caprylic immunoaffinity can be noted. McLaren et al. used Caprylic Acid (CA) in order to total extraction of IgG [10]. Further, in 1999, Dos Santos et al. used this compound for purification of F(ab´)2 antibody part for affording antivenoms, todays, Caprylic Acid (CA) is used in different countries as a method for creation of antivenoms [11].
Caprylic Acid (CA) leads a major part of plasma or serum proteins to sediment and therefore no F(ab´)2 antibody is remained in supernatant, antivenoms which are provided using CA, are more appropriate for production time, albumin/ globulin ratio, turbidity, protein aggregates, electrophoretic pattern and neutralizing potency against venom comparing to AS, on the other hand, CA leads the solution to remain antibody and also leads to destroying of some zoonotic viruses which are able to cause disease in human and horse [12,13].
However, this method needs an extensive dialysis for omitting of CA which is time consuming and creates the risk of contamination with endotoxin generating bacteria; dialysis or ultrafiltration also disrupts complete removing of pepsin which can effect on the antibody stability [12]. In order to solving these problems, recent studies used combinative methods along with CA method which have been helpful in some cases, for instance, Raweerith et al. used CA and cationic ion-exchange chromatography combination in order to prepare equine antivenom, their results confirmed the efficacy of their method, with regards to the above notes, the aim of the present study was to prepare equine antivenom using combination of CA and AS methods as a novel combinative and effective method in improving of F(ab´)2 antivenom [14].
Materials and Methods
Materials
Chemicals and biochemicals were of reagent grade and were from Merck chemicals (Germany) or Sigma Aldrich (St-Louis, MO, USA). Hyper immune monovalent scorpion Plasma was separated from whole blood of production horses from serum and Vaccine Research Institute Ahvaz branch.
Antiserum
Horse hyper-immune plasma (against Hemiscorpius lepturus venom) was provided by Razi Serum and Vaccine Research Institute Ahvaz branch South Western Iran. The pool of horse plasma was obtained following standard immunization schedules. The hyperimmune plasma was stored at 4ºC until purification.
Protein concentration
Protein concentrations were determined by biuret method.
Purity
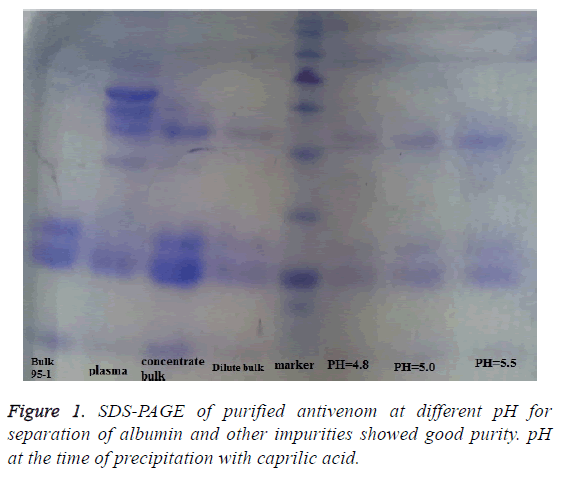
Filtrate samples from the assay described above were analyzed by vertical Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) according to Laemmli using a 10% Polyacrylamide gel under non-reducing conditions (Figures 1-3). All samples (10 μl) were used at concentration of 500 μg/ml. The gel was stained with Coomassie Brilliant Blue R-250 (Bio-RA laboratories).
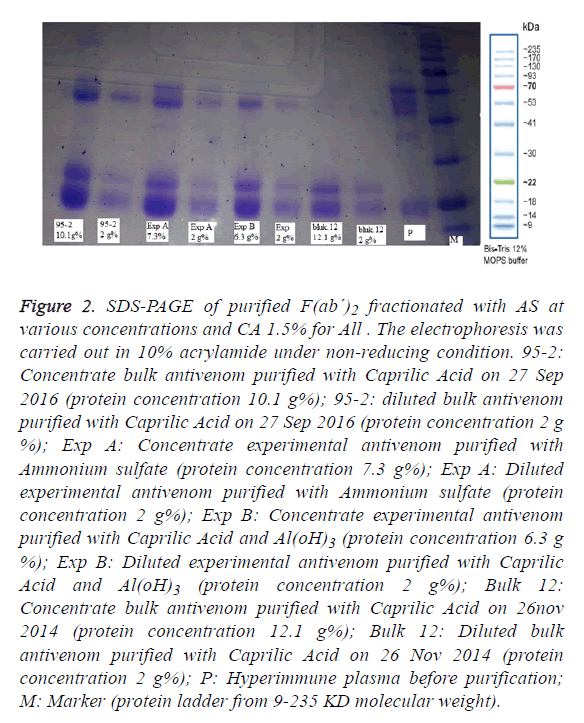
Figure 2: SDS-PAGE of purified F(ab´)2 fractionated with AS at various concentrations and CA 1.5% for All . The electrophoresis was carried out in 10% acrylamide under non-reducing condition. 95-2: Concentrate bulk antivenom purified with Caprilic Acid on 27 Sep 2016 (protein concentration 10.1 g%); 95-2: diluted bulk antivenom purified with Caprilic Acid on 27 Sep 2016 (protein concentration 2 g %); Exp A: Concentrate experimental antivenom purified with Ammonium sulfate (protein concentration 7.3 g%); Exp A: Diluted experimental antivenom purified with Ammonium sulfate (protein concentration 2 g%); Exp B: Concentrate experimental antivenom purified with Caprilic Acid and Al(oH)3 (protein concentration 6.3 g %); Exp B: Diluted experimental antivenom purified with Caprilic Acid and Al(oH)3 (protein concentration 2 g%); Bulk 12: Concentrate bulk antivenom purified with Caprilic Acid on 26nov 2014 (protein concentration 12.1 g%); Bulk 12: Diluted bulk antivenom purified with Caprilic Acid on 26 Nov 2014 (protein concentration 2 g%); P: Hyperimmune plasma before purification; M: Marker (protein ladder from 9-235 KD molecular weight).
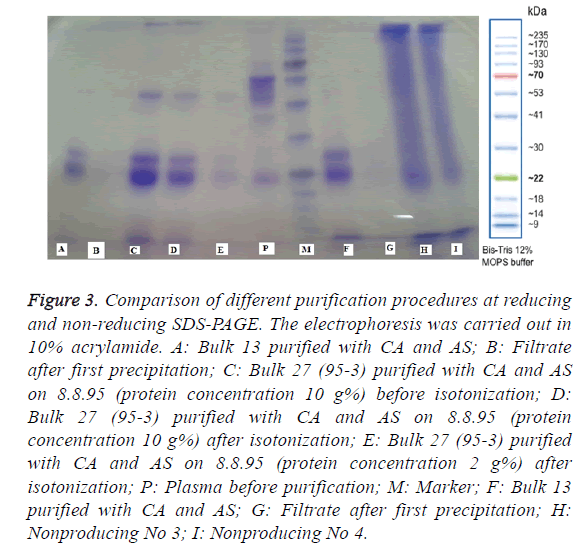
Figure 3: Comparison of different purification procedures at reducing and non-reducing SDS-PAGE. The electrophoresis was carried out in 10% acrylamide. A: Bulk 13 purified with CA and AS; B: Filtrate after first precipitation; C: Bulk 27 (95-3) purified with CA and AS on 8.8.95 (protein concentration 10 g%) before isotonization; D: Bulk 27 (95-3) purified with CA and AS on 8.8.95 (protein concentration 10 g%) after isotonization; E: Bulk 27 (95-3) purified with CA and AS on 8.8.95 (protein concentration 2 g%) after isotonization; P: Plasma before purification; M: Marker; F: Bulk 13 purified with CA and AS; G: Filtrate after first precipitation; H: Nonproducing No 3; I: Nonproducing No 4.
Purification of F(ab’)2 fragment
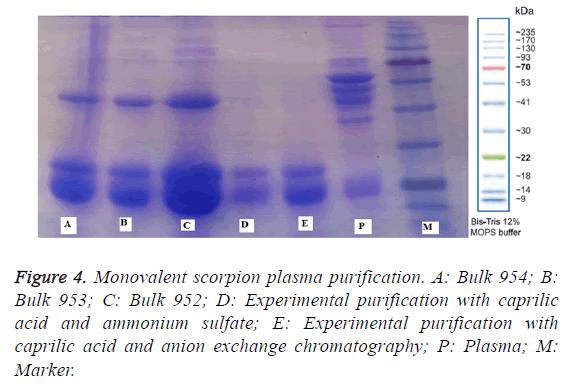
Enzymatic digestion of Fc fragment: Fifty litre batch of monovalent horse plasma against Hemiscorpius lepturus venom was diluted with 50 litres of 0.1 M hydrochloric acid then pH of the diluted plasma was adjusted to 3.5 with 1.5 M hydrochloric acid. The temperature of slurry was fixed at 37°C, then 660 mg of pepsin (Merck) per litre of crude plasma (660 × 50=33000 mg) was added and digestion was carried out for 4 h (Figure 4).
Caprylic acid precipitation
After digestion the pH of slurry was raised to 4.8 with 1 M sodium hydroxide solution and caprilic acid (octanoic) at a final concentration of 1.5% v/v was added and ammonium sulfate to 20% saturation, mixed gently for 60 min at room temperature. The precipitate containing impurities was separated by filter paper and discarded and the filtrate containing F(ab´)2 fragment was collected, dialyzed against CIS buffer to remove caprilic acid and ammonium sulfate and concentrated either with ultra-filter or by precipitation with saturated ammonium sulfate.
Salt precipitation
In our lab since we did not have ultra-filter for concentration of antivenom, we concentrated the product with ammonium sulfate precipitation by adding saturated ammonium sulfate to 50% saturation. Then the precipitate of F(ab´)2 fraction, prepared by CA and ammonium sulfate precipitation was squeezed to a semisolid paste was distributed in dialysis bags and dialyzed against distilled water containing 2.5 g/L phenol for 48-72 h, after removal of ammonium sulfate from antiserum the product was isotonized with the addition of sodium chloride 8 g/L, sodium bicarbonate 1 g/L and phenol 2.5 g/L.
Result
As it is shown in Table 1, caprilic acid noticeably affects the turbidity, when used alone in the absence of ammonium sulfate, for removal of impurities at the 3.5% (v/v) concentration highest turbidity was observed. When caprilic acid is used in combination with ammonium sulfate the turbidity decreased. The concentration of protein in the filtrates of AS and CA precipitated pepsin digested plasma is shown in Table 1, decreases about 13.5%. The combination of AS and CA resulted in more effective protein precipitation, especially at higher reagent concentrations. While the digested plasma control contained the highest protein concentration (52.25 mg/ ml), the filtrate of 20% saturated AS and 1.5% (v/v) CA treated plasma contained the lowest protein concentration (27.63 mg/ ml), indicates that about 53% of digested protein had been precipitated.
| Condition | % AS | 0 | 0 | 10 | 15 | 20 |
|---|---|---|---|---|---|---|
| % CA | 0 | 3.5 | 1.5 | 1.5 | 1.5 | |
| Reaction volume (ml) | 1000 | 1000 | 1000 | 1000 | 1000 | |
| Volume of filtrate | 870 | 774 | 794 | 722 | 792 | |
| Protein of filtrate (mg/ml) | 52.25 | 24.86 | 27.85 | 25.15 | 27.63 | |
| Total protein (G) | 45.457 | 19.241 | 22.827 | 18.158 | 21.88 | |
| Antibody recovery (%) | 100 | 77.4 | 81.96 | 72.34 | 79.2 | |
| Specific activity (unit/mg) | 305.21 | 528.29 | 485.32 | 528.98 | 491.53 | |
| Fold purification | 1 | 1.7 | 1.6 | 1.73 | 1.61 | |
| Turbidity | 0 | 0.6485 | 0.4925 | 0.4533 | 0.4122 | |
Table 1: Quantitative estimation of volume, protein, antibody specific activity, antibody recovery and turbidity of fractions obtained from precipitation at various AS and CA concentrations.
The highest fold-purification of 1.73 was observed at 1.5% CA with 15% AS, but with antibody recoveries of only 72.34%. The highest antibody recovery was seen in 1.5% CA with 20% AS saturation with 1.61 fold-purification.
Discussion
Industrial production of therapeutic antivenom generally includes pepsin digestion of antiserum/plasma and then fractionation of the F(ab´)2 fragment. These methods are performed with the purpose of improving the capacity while decreasing the degree of heterologous protein load obtained by the sufferers, hence decreasing the occurrence of side-effects [14]. Intravenous administration of antivenoms is connected with a high (10-76%) prevalence of negative effects. The most of side-effects are of an anaphylactoid nature [15-17]. Studies performed newly have recommended the incorporation of IgG dimers/aggregates and also Fc gamma receptor. Additional contaminants considered to result in negative effects consist of serum proteins and other fragments or excessive MW aggregates. Consequently, fractionation of antivenom antibody has aimed at clearing the Fc portion by pepsin digestion and also eliminating additional plasma protein impurities or their digestion products by numerous procedures [18,19].
Of most the fractionation methods utilized in industrial production of antivenoms, the most reliable is affinity chromatography [20]. It is often demonstrated that immunochemically pure antibody with a 10-12 fold improvement in potency might be acquired with a single phase of affinity chromatography [21]. Nevertheless, the immunoadsorbant is costly and the recovery of antibody activity is around 30%. As a result, these types of extremely purified antivenoms are not cheap by Scorpion bite patients [22]. In many countries, actually the availability of crude equine antisera does not fulfil the requirement of the envenomed sufferers. Fractionation procedure with considerable lack of antibody activity to acquire purer preparing may deprive Scorpion bite sufferers of any antivenom, pure or else. Because, most of the scorpion bites envenomation instances happen in growing and also poor nations, the price and stability of antivenoms generated are essential aspects [23].
Pepsin digestion has been utilized since 1939 to eliminate the greatly immunogenic Fc of heterologous antibody. Nevertheless, it is essential that pepsin needs to be entirely inactivated or eliminated in subsequent fractionation procedures because the enzyme can influence the stability of the antivenom [14]. It is often demonstrated that aqueous antivenoms are much less stable that those saved in lyophilized type [24]. Nevertheless, lyophilized antivenoms should be solubilized and the solubilization time is long, however time is important in serious envenomed cases. Furthermore, imperfect solubilization of antivenom proteins is irregularly experienced and the particulates might lead to severe side-effects. This was demonstrated that simply changing the enzyme digestion mixture to pH 7.0-7.5 (2 h at 37°C) did not entirely inactivate pepsin. A pH of 8.0 or higher was needed to abolish pepsin activity, a condition additionally detrimental to the antibody [25]. Ultrafiltration utilizing a membrane with MWCO of 50 kDa cannot fully eliminate pepsin, and ionexchange chromatography on Q-Sepharose needed to be utilized to eliminate any residual pepsin from ovine F(ab´)2 solutions [21].
Ammonium sulfate is commonly utilized in large-scale fractional precipitation of IgG or F(ab´)2. The negative aspect of ammonium sulfate precipitation is usually that the F(ab´)2 product needs to be recovered and resolubilized. This method is hard to perform on a significant scale under sterile situations, and endotoxin pollution is usually discovered. The procedure can also involve an important decrease of antibody activity [26,27]. Caprylic acid fractionation has the benefit of precipitating numerous serum proteins or their fragments although leaving the F(ab´)2 in the supernatant. Considerably, the high MW aggregates were not identified in the F(ab´)2 arranged by caprylic acid precipitation. Since alluded to before, the high MW aggregates are considered reliable, at least in part, for the negative effects to intravenously applied antivenom [13].
The risk of viral contamination of biological products is a subject of great concern to regulatory authorities. There are numerous of harmful viruses of equine source: Equine Infectious Anaemia Virus, a lentivirus of the same family as HIV, influenza and Japanese Encephalitis or Venezuelan Equine Encephalitis Virus. It could be useful if the process accustomed to make antivenoms could eliminate or inactivate probably types of virus pollution [28]. In this regard, the fractionation identified here regarding pepsin digestion and also treatment with caprylic acid might inactivate several viruses because of the acidic situation and the detergent action of the organic acid [14]. The elimination of small peptide fragments and/or reagents utilized in the fractional precipitation of antibody is generally performed by dialysis in cellulose bags. This procedure is time-consuming and can obtain several days. It really is expensive in terms of pyrogenfree water, and hazards pollution with endotoxins, that may lead to rejection of the valuable antivenoms [29].
With regards to the above notes, the aim of the present study was to prepare equine antivenom using combination of CA and AS methods as a novel and effective combinative method in improving of F(ab) antivenom. The results of this study showed that using CA and then AS can be effective in extraction of F(ab')2 at high volumes, our results was consistent with results of Eursakun et al. study (2012) who showed this method is effective in antivenom preparation, the difference of results of our study with Eursakun et al. study was the type of antivenom [27]. Their study was on snake poison and ours was on scorpion poison.
Conclusion
The results of the present study showed that the application of CA and AS for antivenom preparation is a simple, economical and fast method, also the high purity of antibody is the most important characteristic of this method and it can be suggested that this method can be used for preparation of antivenom in order to treatment of scorpion poisoned persons, although this matter needs more researches.
Author’s Contribution
All authors have equally contributed to this study.
Conflicts of Interest
There is no conflict of interest.
Funding/Support
This study was financially supported by Department of chemistry, Yazd branch, Islamic Azad University, Yazd, Iran.
References
- Chippaux JP, Goyffon M. Epidemiology of scorpionism: a global appraisal. Acta Tropica 2008; 107: 71-79.
- Forrester MB, Stanley SK. Epidemiology of scorpion envenomation’s in Texas. Veterinary Hum Toxicol 2004; 46: 219-221.
- Abroug F, ElAtrous S, Nouria S, Haguiga H, Touzi N, Bouchoucha S. Serotherapy in scorpion envenomation: a randomised controlled trial. Lancet 1999; 354: 906-909.
- Sofer S, Shahak E, Gueron M. Scorpion envenomation and antivenom therapy. J Pediatr 1994; 124: 973-978.
- Gutiérrez JM, León G, Lomonte B. Pharmacokinetic-pharmacodynamic relationships of immunoglobulin therapy for envenomation. Clin Pharma 2003; 42: 721-741.
- Vazquez H, Chavez-Haro A, Garcia-Ubbelohde W, Mancilla-Nava R, Paniagua-Solis J, Alagon A. Pharmacokinetics of a F (ab')2 scorpion antivenom in healthy human volunteers. Toxicon 2005; 46: 797-805.
- Pepin-Covatta S, Lutsch C, Grandgeorgefi M, Lang J, Scherrmann JM. Immunoreactivity and pharmacokinetics of horse anti-scorpion venom F (ab')2-scorpion venom interactions. Toxicol Appl Pharmacol 1996; 141: 272-277.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol 2003; 41: 937-940.
- Morais J, De Freitas M, Yamaguchi I, Dos Santos M, Da Silva WD. Snake antivenoms from hyperimmunized horses: comparison of the antivenom activity and biological properties of their whole IgG and F(ab')2 fragments. Toxicon 1994; 32: 725-734.
- McLaren R, Prosser C, Grieve R, Borissenko M. The use of caprylic acid for the extraction of the immunoglobulin fraction from egg yolk of chickens immunised with ovine α-lactalbumin. J Immunol Methods 1994; 177: 175-184.
- Dos Santos M, Lima MDI, Furtado G, Colletto G, Kipnis T, Da Silva WD. Purification of F(ab')2 anti-snake venom by caprylic acid: a fast method for obtaining IgG fragments with high neutralization activity, purity and yield. Toxicon 1989; 27: 297-303.
- Otero R, Gutiérrez JM, Rojas G, Núñez V, Dıaz A, Miranda E. A randomized blinded clinical trial of two antivenoms, prepared by caprylic acid or ammonium sulphate fractionation of IgG, in Bothrops and Porthidium snake bites in Colombia: correlation between safety and biochemical characteristics of antivenoms. Toxicon 1999; 37: 895-908.
- Otero R, León G, Gutiérrez JM, Rojas G, Toro MF, Barona J. Efficacy and safety of two whole IgG polyvalent antivenoms, refined by caprylic acid fractionation with or without β-propiolactone, in the treatment of Bothrops asper bites in Colombia. Trans Royal Soc Trop Med Hygiene 2006; 100: 1173-1182.
- Raweerith R, Ratanabanangkoon K. Fractionation of equine antivenom using caprylic acid precipitation in combination with cationic ion-exchange chromatography. J Immunol Methods 2003; 282: 63-72.
- Karlson-Stiber C, Persson H. Antivenom treatment in Vipera berus envenoming-report of 30 cases. J Internal Med 1994; 235: 57-61.
- Ahmed SM, Ahmed M, Nadeem A, Mahajan J, Choudhary A, Pal J. Emergency treatment of a snake bite: Pearls from literature. J Emerg Trauma Shock 2008; 1: 97.
- Theakston R, Warrell D. Crisis in snake antivenom supply for Africa. Lancet 2000; 356: 2104.
- Bleeker WK, Teeling JL, Verhoeven AJ, Rigter GM, Agterberg J, Tool AT. Vasoactive side effects of intravenous immunoglobulin preparations in a rat model and their treatment with recombinant platelet-activating factor acetyl hydrolase. Blood 2000; 95: 1856-1861.
- Chippaux JP. Emerging options for the management of scorpion stings. Drug Design Develop Therapy 2012; 6: 165.
- León G, Segura Á, Gómez A, Hernandez A, Navarro D, Villalta M. Industrial production and quality control of snake antivenoms. Venom Genomics Proteomics 2016; 425-450.
- Jones R, Landon J. Enhanced pepsin digestion: a novel process for purifying antibody F(ab') 2 fragments in high yield from serum. J Immunol Methods 2002; 263: 57-74.
- Laustsen AH, Johansen KH, Engmark M, Andersen MR. Recombinant snakebite antivenoms: A cost-competitive solution to a neglected tropical. Plos Neglected Trop Dis 2017; 1: 14.
- Morais V, Massaldi H. Snake antivenoms: adverse reactions and production technology. J Venomous Animals Toxins Trop Dis 2009; 15: 2-18.
- Gutiérrez JM, Rojas E, Quesada L, León G, Núñez J, Laing GD. Pan-African polyspecific antivenom produced by caprylic acid purification of horse IgG: an alternative to the antivenom crisis in Africa. Trans Royal Soc Trop Med Hygiene 2005; 99: 468-475.
- Dart RC, Seifert SA, Boyer LV, Clark RF, Hall E, McKinney P. A randomized multi-center trial of crotalinae polyvalent immune Fab (ovine) antivenom for the treatment for crotaline snakebite in the United States. Archiv Internal Med 2001; 161: 2030-2036.
- Maria Gutierrez J, León G, Lomonte B, Angulo Y. Antivenoms for snakebite envenomings. Inflammation Allergy Drug Targets (Formerly Current Drug Targets-Inflammation and Allergy) 2011; 10: 369-380.
- Eursakun S, Simsiriwong P, Ratanabanangkoon K. Studies on the fractionation of equine antivenom IgG by combinations of ammonium sulfate and caprylic acid. Toxicon 2012; 60: 1022-1029.
- Burnouf T, Griffiths E, Padilla A, Seddik S, Stephano MA, Gutiérrez J-M. Assessment of the viral safety of antivenoms fractionated from equine plasma. Biologicals 2004; 32: 115-128.
- Al-Abdulla I, Casewell NR, Landon J. Single-reagent one-step procedures for the purification of ovine IgG, F(ab')2 and Fab antivenoms by caprylic acid. J Immunol Methods 2014; 402: 15-22.