ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2014) Volume 25, Issue 3
In vivo study of the role of Tonicity-responsive Enhancer Binding Protein (TonEBP) and its modulation, on biochemical parameters in rats.
1Department of Pharmacology & Clinical Pharmacology, Christian Medical College, Vellore, Tamilnadu, India
2Department of Neurochemistry, Christian Medical College, Vellore, Tamilnadu, India
3Department of Physiology, Christian Medical College, Vellore, Tamilnadu, India
- *Corresponding Author:
- Manoj G Tyagi
Department of Pharmacology & Clinical Pharmacology
Christian Medical College
Bagayam, Vellore-632002
TamilNadu, India
Accepted March 04 2014
The objective of this study was to evaluate the role of tonicity-responsive Enhancer Binding Protein (TonEBP) modulation on biochemical parameters in a rat model. Hypertonicity is traumatic to the cells of all organisms. Cells survive in a hypertonic environment by increasing the transcription of genes whose products catalyze cellular accumulation of compatible osmolytes. Inhibition of this crucial protein and its transduction process can result in deleterious effects on physiology. Hypertonicity in animals was achieved by treating animals with lithium orally, which lead to a condition known as diabetes insipidus with marked hypertonicity. Doxorubicin was used as a tool for the inhibition of TonEBP and related transduction pathway following which the various tests were performed to see the deleterious effects in vivo. On analysis, mean arterial pressure did not change except in the group where doxorubicin was used for inhibiting the TonEBP in lithium treated rats. Aldose reductase enzyme activity, which is one of the markers of hypertonicity was found to be increased in rats treated with lithium. Groups receiving combination therapy showed a decrease in enzyme activity. Serum amino acids analysis revealed an increase in levels of taurine, glycine and serine in lithium treated group and decrease in the groups with combination therapy. In combination group of lithium and doxorubicin, serum creatinine was significantly higher. There were changes as well in the levels of blood electrolytes like sodium, chloride and potassium. Histopathological studies on the kidney showed only mild degeneration in the group treated with lithium along with doxorubicin. Lithium administration develops a hypertonic state, which is evident with the increase in the rate-limiting biosynthetic enzymes— aldose reductase (AR) for sorbitol and also causes overexpression of some amino acids, which are osmoprotective in nature. When the expression of TonEBP was inhibited with doxorubicin in a dose dependent manner it resulted in alterations in the biochemical parameters.
Keywords
TonEBP, Lithium, hyperosmolarity, aldose reductase, doxorubicin, amino acid
Introduction
Water homeostasis and concentration of urine through urinary tubular water reabsorption are vital functions of kidney [1]. Acquired nephrogenic diabetes insipidus and associated polyuria in patients, resulting from lithium treatment, is the most significant effect on sodium excretion. Lithium induced natriuresis lasts for about 1- 2 days and is followed by sodium retention, which lasts for a longer time and then returns to sodium balance [2,3]. During the sodium retention stage there is a state of hypertonicity to which long-term adaptation is achieved by cellular accumulation of organic osmolytes (also called compatible osmolytes) that leads to the lowering of intracellular ionic strength via osmotic replacement [4]. During hypertonic conditions, cellular buildup of compatible osmolytes dampens the activation of caspases and apoptosis. In contrast, if this process is inhibited, in hypertonic culture condition, cell death occurs and in a hypertonic kidney medulla it causes deadly acute renal atrophy [5]. Regulation of the transcription factor; tonicity-responsive enhancer bind ing protein (TonEBP), leads to the accumulation of osmolytes. TonEBP stimulates genes, which codes for transporters and enzymes that catalyze cellular accumulation of organic osmolytes such as, the sodium/ myoinositol cotransporter (SMIT) [6], the sodium/ chloride/betaine cotransporter (BGT1) [7], and aldose reductase (AR) [8]. In hypertonic medulla the abundance of TonEBP and mRNA of SMIT, BGT, and AR is comparatively higher than the renal cortex and other tissues [9]. TonEBP is ubiquitously expressed even in tissues that are not exposed to hypertonic environment, such as the brain, heart, and skeletal muscle [10]. Recent studies have demonstrated that disruption of TonEBP gene results in late gestational lethality and that surviving TonEBP_/_ mice develop a remarkable atrophy of kidney medulla [11]. Infiltration of Macrophages in the skin of rodents after a high salt-diet, suggests that they may regulate the electrolyte homeostasis of this compartment. It has been shown that this buffering mechanism depends on a transcription factor; tonicity enhancer binding protein (TonEBP), which directs vascular endothelial growth factor C (VEGF-C) driven hyperplasia of the lymph capillary network and blocking of which could result in electrolyte accumulation in the skin and an increase in blood pressure [12].
Several other studies indicate the role of different amino acids like taurine [13], glycine [14] and arginine [15] as volume regulatory osmolytes in mammalian cells. Hypokalemia causes a significant decrease in the tonicity of the renal medullary interstitium associated with reduced expression of sodium transporters in the distal tubule [16]. In vitro studies in mice inner medullary collecting duct cells showed increase in osmolality from 300 to 500 mosmol/kg by adding NaCl, which increases TonEBP mRNA [17]. Mature cardiac myocytes have a restricted proliferative potential. Heart failure can result from accumulation of dead cardiomyocytes, which is induced by hypoxia, different types of stress and cardiotoxic drugs. Among them Doxorubicin (Dox) an anti-tumor drug is well known to cause cardiotoxicity [18,19]. Dox induced cardiotoxicity could be induced by various possible mechanism such as, production of reactive oxygen species, perturbation of calcium handling, and selective inhibition of cardiac muscle-specific gene expression [18,20,21]. Some recent studies indicate that Dox activates proteasomemediated proteolysis of transcriptional property of TonEBP that results in the disorder of cardiac gene expression in cultured cardiomyocytes and may affect cardiac parameters [22,23]. Hence, in this study, with the help of lithium, a hypertonic state was created in rats that would eventually increase the transcriptional factor, TonEBP expression and its related transduction products like enzymes and amino acids. The anticancer drug doxorubicin was used as a tool to study the effects on TonEBP and the overall effects on cardiovascular function and on the biochemical parameters in different groups of animals.
Materials and Methods
Animals
Healthy, male albino Wistar rats each weighing 250- 300 g were used for this study. The rats were housed in polypropylene cages and maintained under standard conditions (12 h light and dark cycles, at 25±3ºC and 35-60% humidity). Standard pelletized feed and tap water were provided ad libitum. The study was approved by the Institutional Animal Ethical Committee of Christian Medical College, Vellore, India, registered under CPCSEA, India (Registration No. 88/1999/CPCSEA). All the procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals.
Drugs and chemicals
The drugs, which were used in the study, were normal saline (0.9%), doxorubicin hydrochloride, heparin obtained from the Hospital Pharmacy of the Christian Medical College and Hospital, Vellore, India. Other drugs like lithium chloride, kit for electrolyte estimation and chemicals of analytical grade were obtained from Sigma Aldrich India, Fisher Scientific India and Abbott India Limited.
Study Design
Animals were divided into 6 groups with 6 animals in each group (n=6). Group 1 served as controls, which were given 1g/Kg/day of saline. Group 2 was treated with 3 meq lithium/Kg every alternative day orally for 14 days. Groups 3 and 4 received graded doses of doxorubicin hydrochloride i.e a total dose of 3mg/Kg and 6mg/Kg i.p in equally divided doses every alternative day for a period of 2 weeks respectively, which were lower than the cardiotoxic [24] and nephrotoxic doses [25,26]. Rats in Groups 5 and 6 received combination of lithium and two different graded doses of doxorubicin hydrochloride respectively in the same above-mentioned regimen for a similar period of time. Experiments were continued for 14 days and on day 15 mean arterial pressures were analysed following which blood was collected for estimation of other biochemical parameters and kidneys were removed for histopathological analysis.
In vivo experiments
Rats were anesthetized with either thiopentone (40 mg/kg) or ketamine (75–100 mg/kg) given intraperitoneally. The carotid artery was cannulated and the animal was heparinized with 0.25 ml of 1000 IU/ml of Heparin [27]. The cannula was then connected to a three-way stopcock, which was finally connected to the pressure transducer and a syringe filled with heparinized saline. Heparinized saline was used to apply a positive pressure and maintain it at the baseline value. The three-way stopcock worked as a passage connecting the pressure transducer and carotid cannula. With the help of data acquisition system the mean arterial pressure recordings were noted. After the arterial pressure studies were over the blood of the rats were collected for biochemical analyses.
Biochemical Methods
Electrolyte analysis
Immediately after cannulation, 0.25 ml of blood was withdrawn from the carotid artery and kept aside for reinfusion later. Another 0.25 ml was withdrawn and injected into the cartridge (iSTAT, EC8+ cartridge, Abbott, USA) for immediate electrolyte (Na+2, K+ and Cl- ) analysis with the help of automated analyzer [28].
Analysis of amino acids in Plasma
Plasma samples were deproteinized with equal volumes of 5% TCA for 1 hour at 25oC and centrifuged at 450g for 10 minutes. The deproteinized supernatants were diluted 5-fold with 67mM sodium citrate buffer pH 2.2, filtered through 0.22μm Millipore filters. Amino acids like taurine, glycine and arginine were analyzed in 10μl of the filtrate by High Performance Liquid Chromatography by adsorption to a strong cation exchanger and elution done according to their isoelectric pH over the pH range 3.2 to 10.0. The HPLC system used was Shimadzu LC-20AD [29].
Aldose reductase enzyme estimation
Blood was drawn from the rats into anticoagulant tubes after that Red blood cell (RBC) were separated by centrifugation, washed thrice with saline, and stored until further analysis. A 10% erythrocyte suspension was made by adding 50 mM sodium phosphate buffer, pH 7.4, containing 150 mM NaCl. The suspension was lysed by repeated freezing and thawing (three cycles) and centrifuged to remove insoluble material, if any. Aldose reductase activity was measured spectrophotometrically using an appropriately diluted hemolysate [30] using T60 LAB INDIA UV-VIS spectrophotometer. The assay mixture in 1 ml contained 50 μmol potassium phosphate buffer pH 6.2, 0.4 mmol lithium sulfate, 5 μmol 2-mercapto ethanol, 10 μmol DLglyceraldehyde, 0.1 μmol NADPH andenzyme preparation. The assay mixture was incubated at 37 °C and initiated by the addition of NADPH at 37 °C. The change in the absorbance at 340 nm due to NADPH oxidation was recorded.
Serum creatinine estimation
The UV-VIS Spectrophotometry method of serum estimation by Jaffe’s reaction involves preparation of calibration curve using standard creatinine solution in water. Aliquots in the range of 2-10 ppm were prepared to which 2.5ml of the alkaline picrate solution, containing 1% picric acid and 10% sodium hydroxide was added and the final dilutions were made with distilled water. Absorbance of the resulting solutions was measured at 496 nm [31].
Histopathological examination
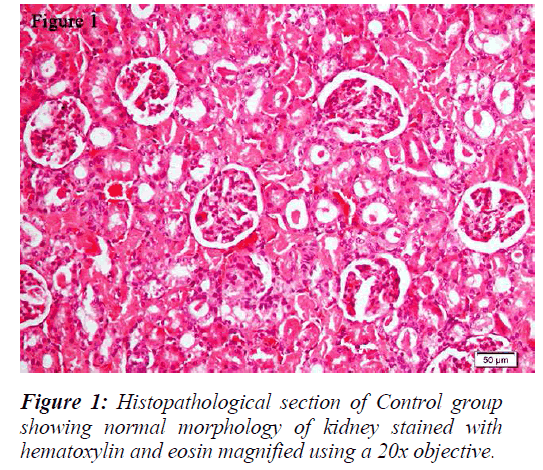
The kidneys were sectioned longitudinally in two halves and were kept in 10% neutral formalin solution [32]. Both kidneys were processed and embedded in paraffin wax and sections were taken using a microtome. The sections were stained with hematoxylin and eosin and examined under Olympus BX43 light microscope. Digital pictures were taken with the help of DP21 camera.
Statistical analysis
The data obtained was analyzed using one-way ANOVA followed by Tukey HSD for multiple comparison tests. P < 0.05 was considered significant. SPSS statistics (version 17.0) was used for statistical analysis.
Results
Arterial Pressure evaluation
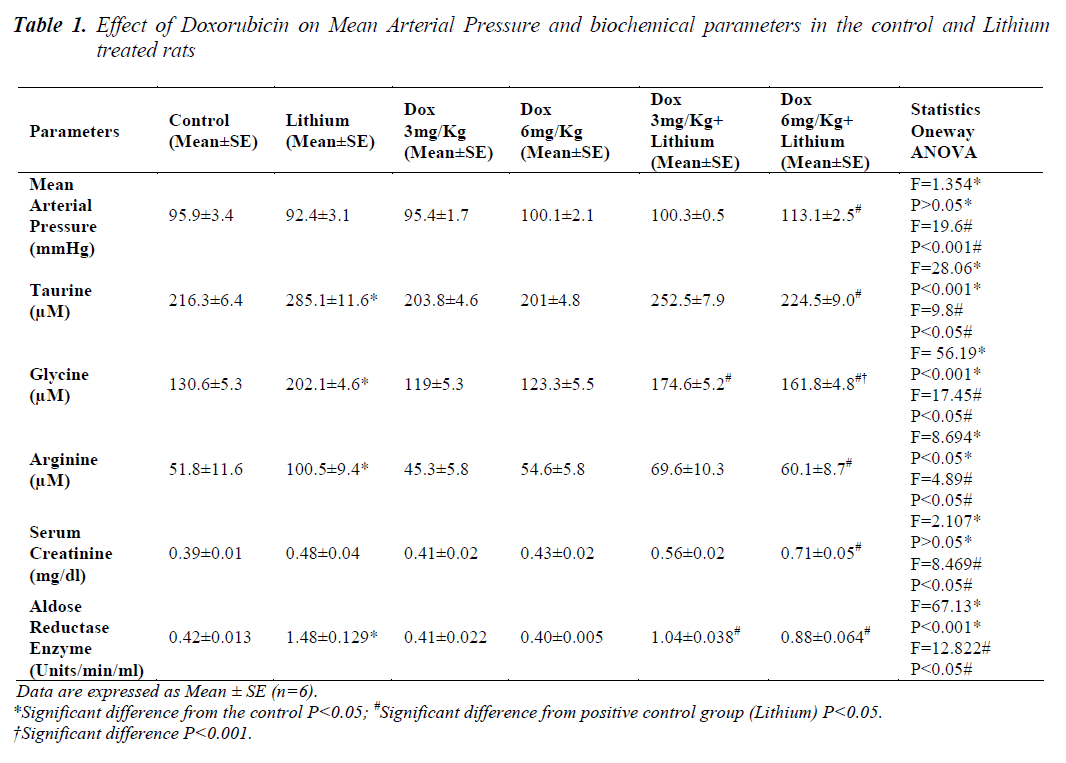
The results of comparison between the groups are given in Table 1.Animals in groups receiving lithium, Dox 3mg/Kg and Dox 6mg/Kg, at the end of 14 days, showed that there was no change in the mean arterial pressure when compared to control animals. Only in group 6 animals, receiving 6mg/Kg along with lithium, showed significant increase (p<0.05) in mean arterial pressure when compared to lithium treated animals alone.
Electrolytes and amino acid estimation
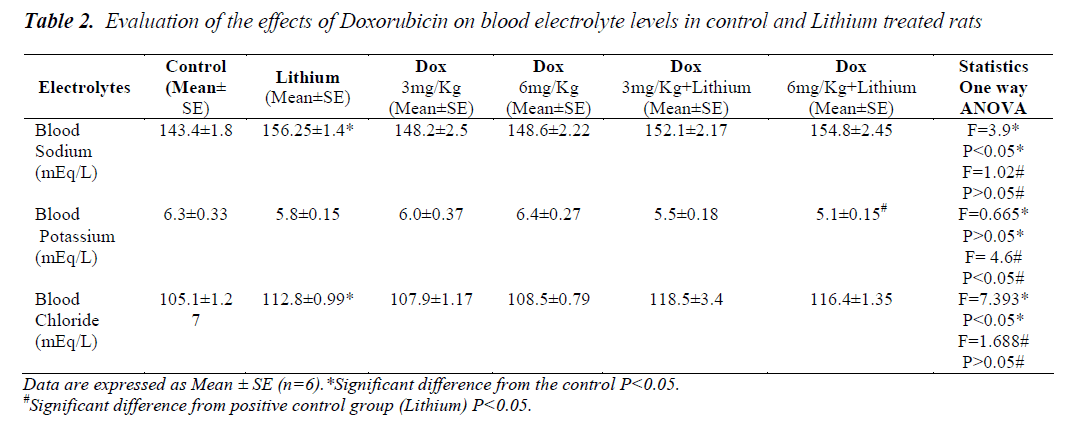
In lithium treated animals when compared to control animals (P<0.05) there was an increase in the sodium levels but it seemed to be in the normal range. Compared to lithium, group 5 and 6 did not have any changes. Similarly in chloride levels, animals showed increase (p<0.05) only on lithium treatment when compared to control. Combination treatment of lithium and doxorubicin did not show any difference when compared to lithium. A significant reduction in potassium level was noticed only in the group receiving Dox 6mg/Kg along with lithium shown in Table 2. Levels of all the three amino acids; taurine, glycine and arginine were significantly higher in lithium treated animals (p<0.05) when compared to normal group, Table 1. A significant reduction in the level of all the three amino acids was also seen in group 6. A significant decrease in the levels of glycine was found only in group 5.
Serum creatinine estimation
There were no changes as described in Table 1 found in serum creatinine levels in groups 2, 3 and 4 when compared to control animals but in combination therapy of Dox 6mg/Kg along with lithium it was significantly higher (p<0.05) compared to lithium the positive control.
Aldose reductase enzyme activity
Aldose reductase enzyme activity was significantly higher (p<0.05) only in the lithium treated group compared to controls. Both the doses of doxorubicin i.e. 3mg/Kg and 6mg/Kg along with lithium caused a significant reduction in the enzyme activity compared to Lithium as depicted in Table 1.
Histopathological examination
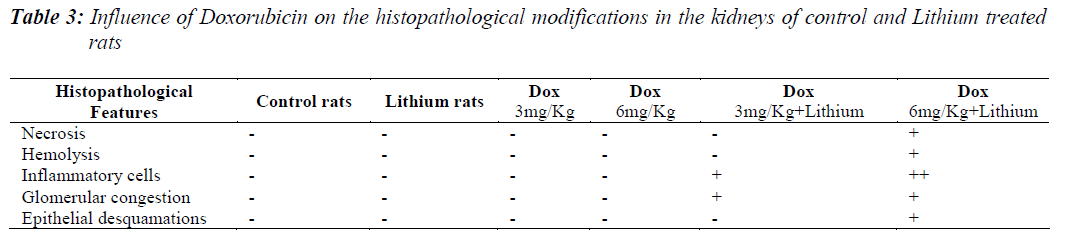
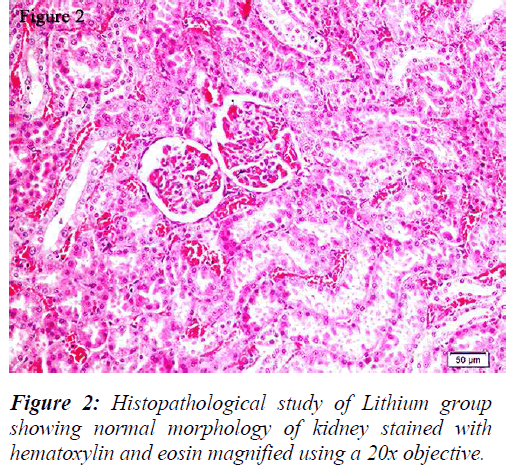
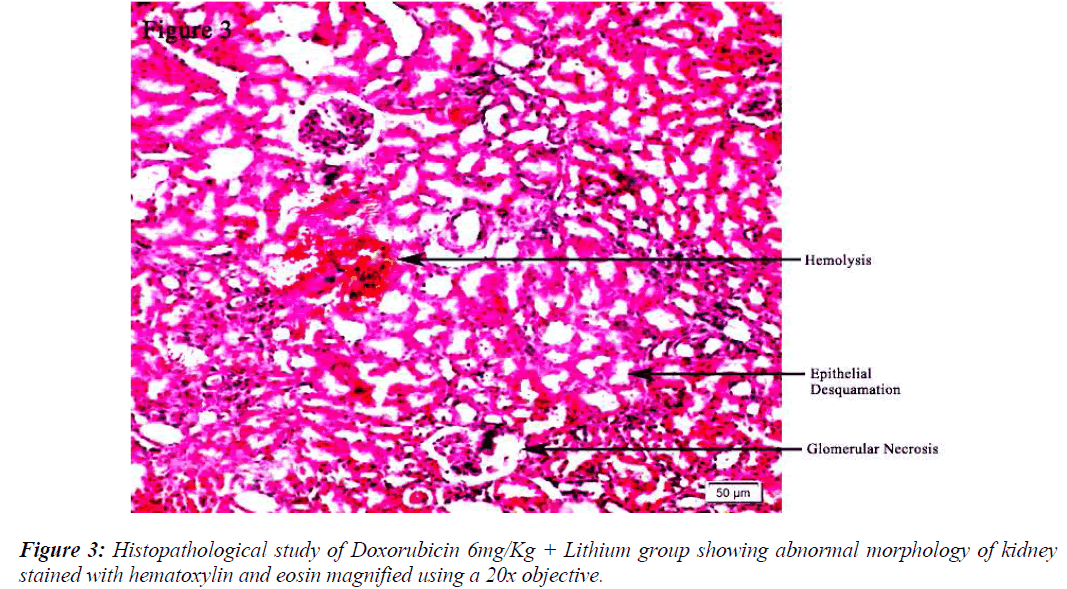
All the groups, except in doxorubicin 6mg/Kg treated along with lithium group, rats’ kidneys showed normal glomerular and tubular histology (Refer Table 3, Figure 1) whereas in group 6 it was found to cause only mild glomerular, peritubular and vascular congestion which resulted in the presence of inflammatory cells in kidney sections as shown in Table 3 and Figure 3.
Discussion
This study shows that lithium-induced polyuria imposes a hyperosmolar state in the body, which could be due to the changes happening in the levels of different electrolytes such as sodium and potassium. In response to this there is upregulation of TonEBP that has already been suggested in previous studies [33]. TonEBP leads to increase in the transcription of various organic osmolytes and other enzymes, e.g., AR, which ultimately synthesizes sorbitol, an organic osmolyte, from glucose and is osmoprotective in nature [34]. Various studies have also suggested that there is an upregulation of taurine transporters in cells, which leads to accumulation of taurine in tissues. Taurine contributes in many physiological functions, such as modulation of ion movement, regulation of intracellular osmolality, conjugation with bile acids, detoxification and membrane stabilization [35,36]. Taurine has also been reported to prevent cell injury induced by stimulations, such as hypoxia [37]. Taurine comprises 50% of the cardiac free amino acid pool and is present in the myocardial tissues in the concentration of 11-38 μM/g [38]. It plays an important role in the regulation of ion flow along with cardiac contractility and regulation of membrane excitability [39]. There are evidences that not only taurine but other amino acids, such as arginine and glycine also support their accumulation during hyperosmolality as organic osmolytes [13,14,15]. Our study also supports this theory by showing a significant increase in the levels of AR and amino acids taurine, arginine and glycine in rat plasma of lithium treated animals. This indicates that during hypertonic state the body tries to blunt the osmotic stress by not only increasing the transporters in cells but also the blood levels of amino acids, which with the help of transporters gets accumulated into the tissues and provide safety from osmotic stress. The inhibition of production of such elements during osmotic overload could be dangerous. As we have shown in our research; in animals where the expression of TonEBP was inhibited by doxorubicin, the levels of these enzymes and amino acids were attenuated. Treatment with doxorubicin 6mg/Kg in polyuric animals resulted in changes in mean arterial pressure and histopathology which showed an increase in the blood pressure and mild nephrotoxic effect respectively. The changes happening in group 6 also suggests the possible role of TonEBP in cardiotoxicity of doxorubicin and its association with taurine. The mechanism by which Dox affects the heart remains to be fully elucidated but oxidative stress from Dox metabolites may be an important cause [40]. It has been reported that treatment with the antineoplastic drug Dox, decreases the expression of the TauT gene in cultured cardiomyocytes isolated from neonatal rat heart. The protein level of TonEBP was reduced by Dox treatment. In addition, the reduction in TonEBP protein content was suppressed by proteasome inhibitors. Thus the Dox-enhanced degradation of TonEBP results in reduced TauT expression in the cardiomyocytes hence signifying a role in the cardiovascular system.
On the other hand there was a significant increase in the serum creatinine levels as per our results, which is an indicator of renal function. During lithium treatment there is an osmotic overload on kidneys which is managed by the osmolytes produced by transcriptional factor TonEBP i.e. group 2 did not show any degenerative changes (Table 3 and Figure 2) but when the same process was inhibited by higher dose doxorubicin in lithium treated animals we could find mild morphological changes in rat renal tissue. This finding supports the role of TonEBP in the development and maintenance of kidney morphology and the inhibition of transcriptional response to hypertonic stress could lead to renal atrophy [41].
Electrolyte imbalance due to lithium therapy could be the reason for hypertonicity as per our results depicting the increase in the levels of sodium and chloride. Levels of potassium did not change with increase in the hypertonicity but it could also play a role in the development of hypertonicity. A moderate hypokalemia was noticed in the animals that were exposed to doxorubicin along with lithium therapy (group 6) suggesting that there is a possible correlation between the levels of potassium ion and expression of TonEBP. Previous studies have also demonstrated that the abundance of TonEBP decreased significantly in the outer and inner medullas of hypokalemic rats [42].
Conclusion
The results of this in vivo study shows that TonEBP plays an important role in the proper functioning and maintenance of cardiovascular and renal functions and that the modulation of this transcription factor can lead to a disturbance in homeostasis, in rat model. Most of the published works establishing the role TonEBP has been done either on cultured cells or is limited to the effects on animal kidneys only. Therefore, this lithium treated animal model could be beneficial for studying the role of osmotic stress and related signal transduction in vivo, most importantly, where we can study the effects on the specific tissues as well as on various biochemical processes which is not possible with the help of cell culture studies.
Acknowledgments
We are thankful to the Institutional Review Board, Christian Medical College, Vellore for providing the financial support for the study. The author is also thankful to Mr. Anand Ramasamy (J.R.F.), Department of Pharmacology and Clinical Pharmacology, Christian Medical College, Vellore for his help during the course of this study.
References
- Umenishi F, Schrier RW. Hypertonicity induced aq- uaporin-1 (AQP1) expression is mediated by the activa- tion of MAP kinase pathways and hypertonicity re- sponsive element in the AQP-1 gene. J. Biol. Chem 2003; 278: 15765-15770.
- Lazarus JH, Collard KT. Endocrine & metabolic effects of lithium. In: John.H. Lazarus editor. Lithium and the kidney. 2nded. New York: Plenum Publishing Corpora- tion 1986; p. 163-182.
- Bateman AM, Larner AJ, McCartney SA et al. Rhab- domyolysis associated with lithium-induced hyperos- molal state. Nephrol Dial Transplant 1991; 6: 203-205.
- Kwon MS, Lin SW, Kwon HM. Hypertonic stress in kidney: A necessary evil. Physiology 2009; 24: 186-191.
- Kultz D. Hyperosmolality triggers oxidative damage in kidney cells. PNAS 2004; 101: 9177-9178.
- Rim JS, Atta MG, Dahl SC et al. Transcription of the sodium/myo-inositol cotransporter gene is regulated by multiple tonicity-responsive enhancers spread over 50 kilobasepairs in the 5-flanking region. J. Biol. Chem 1998; 273: 20615-20621.
- Miyakawa H, Rim JS, Handler JS, Kwon HM. Identifi- cation of the second tonicity-responsive enhancer for the BGT-1 gene. Biochim. Biophys. Acta 1999; 1446: 359-364.
- Ko BC, Ruepp B, Bohren KM et al. Identification and characterization of multiple osmotic response se- quences in the human aldose reductase gene. J. Biol. Chem 1997; 272: 16431-16437.
- Na KY, Woo SK, Lee SD, Kwon HM. Silencing of TonEBP/NFAT-5 transcriptional activator by RNA in- terference. J. Am. Soc. Nephrol 2003; 14: 283-288.
- Trama J, Lu Q, Hawley RG, Ho SN. The NFAT- Related Protein NFATL1 (TonEBP/NFAT5) Is Induced Upon T Cell Activation in a Calcineurin-Dependent Manner. J. Immunol 2000; 165: 488-494.
- Maouyo D, Kim JY, Lee SD et al. Mouse TonEBP/NFAT5: expression in early development and alternative splicing Am. J. Physiol 2002; 282: F802- 809.
- Muller S, Quast T, Schroder A et al. Salt-dependent Chemotaxis of Macrophages. PLoS ONE 2013; 8: e73439.
- Law RO. Comparative Biochemistry and Physiology Part A: Physiology 1991; 99: 263-277.
- Dawson KM, Collins JL, Baltz JM. Osmolarity- Dependent Glycine Accumulation Indicates a Role for Glycine as an Organic Osmolyte in Early Preimplanta- tion Mouse Embryos. Biology of reproduction 1998; 59: 225-232.
- Aoki E, Takeuchi IK. Immunohistochemical Localiza-tion of Arginine and Citrulline in Rat Renal Tissue. The Journal of Histochemistry & Cytochemistry 1997; 45: 875-881.
- Jeon US, Han KH, Park SH et al. Downregulation of renal TonEBP in hypokalemic rat. Am J Physiol Renal Physiol 2007; 293: F408-415.
- Gallazzini M, Ferraris JD, Kunin M et al. Neuropathy target esterase catalyzes osmoprotective renal synthesis of glycerophosphocholine in response to high NaCl. Proc Natl Acad Sci U S A 2006; 103: 15260-15265.
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol 2004; 56: 185-229.
- Singhal PK, Iliskovic N. Current Concepts: Doxorubi- cin-Induced Cardiomyopathy. N. Engl. J. Med 1998; 339: 900-905.
- Arai M, Tomaru K, Takizawa T et al. Sarcoplasmic Reticulum Genes are Selectively Down-regulated in Cardiomyopathy Produced by Doxorubicin in Rabbits. J. Mol. Cell. Cardiol 1998; 30: 243-254.
- Ito H, Miller SC, Akimoto H et al. Evaluation of mRNA levels by the polymerase chain reaction in small cardiac tissue samples. Proc. Natl. Acad. Sci. U. S. A 1990; 87: 4275-4279.
- Ito T, Fujio Y, Takahashi K, Azuma J. Degradation of NFAT5, a Transcriptional Regulator of Osmotic Stress- related Genes, Is a Critical Event for Doxorubicin- induced Cytotoxicity in Cardiac Myocytes. The journal of biological chemistry 2007; 282: 1152-1160.
- Pradhap T, Ashokan M, Tyagi MG. Evaluation of TonEBP modulation on heart rate and biochemical pa- rameters. Journal of Medicine and Medical Sciences 2012; 3: 409-414.
- Iqbal M, Dubey K, Anwer T, Ashish A, Pillai KK. Pro- tective effects of telmisartan against acute doxorubicin- induced cardiotoxicity in rats. Pharmacological Reports 2008; 60: 382-390.
- Rashid S, Ali N, Nafees S et al. Alleviation of doxoru- bicin-induced nephrotoxicity and hepatotoxicity by chrysin in Wistar rats. Toxicol Mech Methods 2013; 23: 337-345.
- Mansour MA, El-Kashef HA, Al-Shabanah OA. Effect of captopril on doxorubicin-induced nephrotoxicity in normal rats. Pharmacological Research 1999; 39: 233-237.
- Parasuraman S, Raveendran R. Measurement of inva- sive blood pressure in rats. J Pharmacol Pharmacother 2012; 3: 172-177.
- Subramanian RK, Sidharthan A, Maneksh D et al.Normative data for arterial blood gas and electrolytes in anesthetized rats. Indian J Pharmacol 2013; 45: 103-104.
- Ishida Y, Fujita T, Asai K. New detection and separa- tion method for amino acids by high-performance liq- uid chromatography. J Chromatography 1981; 204:143-148.
- Reddy GB, Satyanarayana A, Balakrishna N et al.Erythrocyte aldose reductase activity and sorbitol levels in diabetic retinopathy. Molecular Vision 2008; 14: 593-601.
- Shah M , Varshney P, Patel P, Rohit M. Comparison of uv-visible spectrophotometric and colorimetric method for estimation of serum creatinine by Jaffe’s alkaline picrate method. World journal of pharmacy and phar- maceutical sciences 2013; 2: 2710-2715.
- Ogeturk M, Kus I, Colakoglu N et al. Caffeic acid phenethyl ester protects kidneys against carbon tetra- chloride toxicity in rats. J Ethnopharmacol 2005; 97: 273-280.
- Han KH, Woo SK, Kim WY et al. Maturation of TonEBP expression in developing rat kidney. Am J Physiol Renal Physiol 2004; 287: F878-885.
- Burg MB, Ferraris JD, Dmitrieva NI. Cellular Re- sponse to Hyperosmotic Stresses. Physiol Rev 2007;87: 1441-1474.
- Azuma J, Sawamura A, Awata N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Jpn. Circ. J 1992; 56: 95-99.
- Schaffer S, Takahashi K, Azuma J. Role of osmoregu- lation in the actions of taurine. Amino Acids 2000; 19: 527-546.
- Takahashi K, Ohyabu Y, Solodushko V et al. Taurine renders the cell resistant to ischemia-induced injury in cultured neonatal rat cardiomyocytes. J. Cardiovasc. Pharmacol 2003; 41: 726-733.
- Huxtable RJ: Physiological actions of Taurine. Physiol Rev 1992, 72: 101-163.
- Schaffer S, Azuma J, Takahashi K, Mozaffari M. Why is Taurine cytoprotective? Adv Exp Med Biol 2003, 526: 307-321.
- Liu X, Chua CC, Gao J et al. Pifithrin-α protects against doxorubicin-induced apoptosis and acute car- diotoxicity in mice. Am J Physiol Heart Circ Physiol 2004; 286: H933–939.
- López-Rodríguez C, Antos CL, Shelton JM et al. Loss of NFAT5 results in renal atrophy and lack of tonicity- responsive gene expression. PNAS 2004; 101: 2392-2397.
- Jeon US, Han KH, Park SH et al. Down regulation of renal TonEBP in hypokalemic rats. Am J Physiol Renal Physiol 2007; 293: F408-415.