ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 11
Influence of anti-infection therapy on changes of dendritic cells in rabbits with buccal VX2 cancer accompanied by infectious inflammation
1Department of Oral and Maxillofacial Surgery, Stomatological Hospital, Zunyi Medical College, Zunyi, PR China
2Department of Oral and Maxillofacial Surgery, Guizhou Provincial Hospital, Guiyang, PR China
#These authors contributed equally to this work
- *Corresponding Author:
- Nini Zhang
Department of Oral and Maxillofacial Surgery
Stomatological Hospital, Zunyi Medical College, PR China
Accepted date: March 23, 2017
Background: This study aimed to investigate the influence of anti-infection therapy on the HLA-DR and CD86 expression, maturation and function of the dendritic cells in rabbits with buccal VX2 cancer accompanied by infectious inflammation.
Methods: Inoculation of VX2 cancer cells, mechanical trauma, and intake of high glucose viscous milk were administered to induce buccal VX2 cancer and wound infection in rabbits. Animals were divided into 3 groups: Group A: buccal cancer rabbits with infectious inflammation received intramuscular injection of procaine and penicillin as well as intragastrical injection of tinidazole for consecutive 3 days; Group B: buccal cancer rabbits with infectious inflammation received intragastrical and intramuscular injection of normal saline for 3 days; Group C: buccal cancer rabbits without infection received intragastrical and intramuscular injection of normal saline for 3 days. The Peripheral Blood Mononuclear Cells (PBMCs) were incubated with the supernatant from cancers at different statuses to induce their differentiation into DCs.
Results: Flow cytometry showed the expression of CD86 and HLA-DR was the highest in Group C and the lowest in Group B in PBMCs after incubation with the supernatant from cancers, and the expression of CD86 and HLA-DR was the highest in Group A and the lowest in Group B in cancer tissues.
Conclusion: Anti-infection therapy can significantly increase the expression of CD86 and HLA-DR (markers of DCs) in the buccal cancer rabbits with infectious inflammation and is helpful for the control of infection in the cancer as well as for the maturation and function of DCs.
Keywords
VX2 cancer, Infection, Dendritic cells.
Introduction
Malignant oral and maxillofacial tumors have a high incidence worldwide, and more than 80% of them are Oral Squamous Cell Carcinoma (OSCC) [1]. OSCC is a common oral malignancy. The incidence of OSCC is increasing over year [2]. OSCC is usually accompanied by mild to moderate local inflammation, but whether the local inflammation may affect the immune status of immune cells in cancer is still poorly understood. In clinical practice, clinicians usually encounter patients with poor oral health, which together with mechanical injury may cause infectious inflammation of different extents in cancer. The persistent stimulation of stimulating factors and the change in normal regulatory mechanism due to tumor inflammation may cause “uncontrollable inflammation”, which makes the tumor microenvironment more complex. Thus, to investigate the pathogenesis of OSCC and to block the specific mechanism are crucial for the prevention and treatment of OSCC, and have been a focus in studies on OSCC. In this study, buccal cancer with infectious inflammation was induced in rabbits, and the inflammation was evaluated in the presence and absence of anti-infection treatment. In addition, the supernatant of OSCC was used to induce the differentiation of Peripheral Blood Mononuclear Cells (PBMCs) from healthy rabbits into Dendritic Cells (DCs) and the expression of Major Histocompatibility Complex (MHC) II HLA-DR and costimulatory molecule CD86 was measured in these cells and in cancer tissues.
Materials and Methods
Materials
Animals: VX2 cancer bearing rabbit was purchased from the Experimental Animal Center of Sun Yat-sen University. New Zealand white rabbits (n=35) aged 2-3 months (male or female) and weighing 1.5-2.0 kg were purchased from the Medicine and Biology Research Center of Zunyi Medical College (License Number: SYXK (Qian) 2011-003).
Drugs: Procaine penicillin injection (Shanghai Quakers veterinary drug factory; batch number: 090052629) and tinidazole tablets (Chongqing Kerui Pharmaceutical Group Co., Ltd.; batch number: H20056192) were used in this study.
Reagent: Fluorescein Isothiocyanate (FITC) conjugated HLADR antibody, FITC conjugated IgG2a antibody, Phycoerythrin (PE) conjugated CD86 antibody, PE conjugated IgG1 antibody (BD company, USA), fetal bovine serum (Gibco, USA) and RPMI1640 (Hyclone, USA) were used in the present study.
Establishment of VX2 cancer model with infectious inflammation in rabbits
VX2 tumor was collected from the limb of cancer-bearing rabbit and VX2 cancer tissues were harvested and inoculated into the cheek of healthy rabbit. At 14 days, cancer was observable. Then, the superficial mucosa was carefully collected from the cancer and a lesion sized 1.0 cm × 1.0 cm formed. The rabbits were fed with sugar rich viscous milk (milk: water: sugar=50:30:20) for 3 days. At 17 days (3 days after lesion induction), ulcer formed at the lesion site, accompanied by evident errhysis and red edema. These suggested that VX2 cancer with infectious inflammation was successfully induced in rabbits. Animals were divided into 3 groups: Group A: VX2 cancer rabbits with infectious inflammation (n=12) received intramuscular injection of procaine penicillin at 22000 U/kg and intragastrical injection of tinidazole at 150 mg/kg for consecutive 3 days (once daily); Group B: VX2 cancer rabbits (n=12) with infectious inflammation (n=12) received intramuscular injection of normal saline (1 ml) and intragastrical injection of normal saline (20 ml) for 3 consecutive days (once daily); Group C: VX2 cancer rabbits (n=12) without infection(n=12) received intramuscular injection of normal saline (1 ml) and intragastrical injection of normal saline (20 ml) for 3 consecutive days (once daily).
Histological examination of the cancer
On day 20, animals were sacrificed, and the cancer was collected. Then, 1/4 of cancer tissues was separated and processed for HE staining.
Separation of PBMCs
A healthy rabbit was anesthetized and placed on a surgical table in a supine position. After removal of hair on the precordial region and skin sterilization, percutaneous puncture was performed in the precordial region with a 20 ml syringe, and blood was collected and anti-coagulated with heparin. PBMCs were separated by density gradient centrifugation. Cells were counted under an inverted phase contrast microscope, and the cells density was adjusted to 2 × 106/ml. Detection of cell viability: cell suspension (9 drops) was mixed with 0.4% trypan blue solution (1 drop), and viable cells were counted. The cell viability was determined as follow: viability=(number of viable cells/number of total cells) × 100%. The cell viability was 95.7% in this study. Then, cell suspension was added to 6-well plate (1 ml/well) and cells were incubated in an environment with 5% CO2 at 37ºC for 3 h. The supernatant was removed and the remaining cells were PBMCs.
Culture of DCs from PBMCs
The cancer was collected from each rabbit. Then, 1/2 of cancer tissues were homogenized with serum-free RPMI1640 at 200 g/L, followed by centrifugation at 3000 r/min for 20 min (2 times). The supernatant was collected and filtered through a 0.22 μm filter for sterilization (3 times). The supernatant was then used to treat PBMCs. Cells were divided into 3 groups: Group A: cells were incubated in RPMI1640 containing 10% FCS (2 ml), antibiotics and 40% supernatant; Group B: cells were incubated in RPMI1640 containing 10% FCS (2 ml) and 40% supernatant; Group C: RP MI1640 containing 10% FCS (2 ml) and 40% supernatant from cancer. Half medium was refreshed once every 2 days, cells were observed under an inverted microscope and images were captured. Cells were cultured for 6 days. Cells in Group C were also harvested for electronic microscopy.
Detection of HLA-DR and CD86 expression of DCs
The cancer was collected from each rabbit and 1/4 of cancer tissues were processed for single cell suspension (1 × 106 cells/ml) and the expression of HLA-DR and CD86 was detected by flow cytometry. Cells were cultured for 6 days, and then the expression of HLA-DR and CD86 was detected by flow cytometry according to manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed with SPSS version 21.0. Comparisons were conducted with one way analysis of variance among groups, and paired comparisons were performed with LSD-t test. A value of P<0.05 was considered statistically significant.
Results
Macroscopic and microscopic observation of buccal VX2 cancer
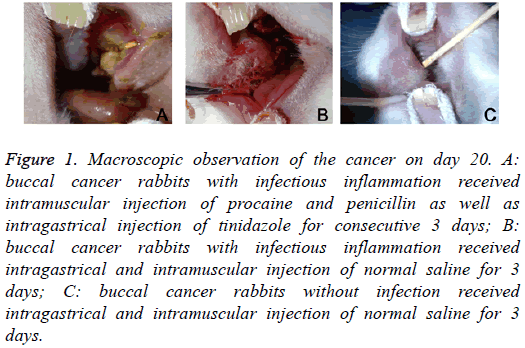
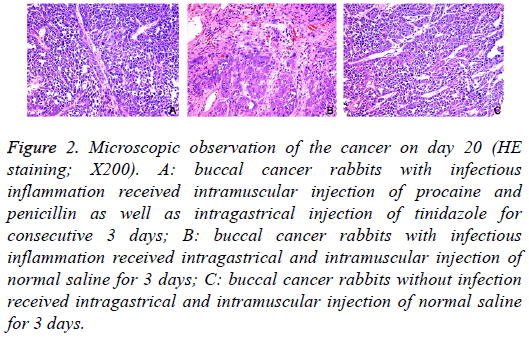
On day 20, macroscopic observation showed: in Group A, the cancer became cankerous, but there was no evident erosion or bleeding; in Group B, evident erosion and bleeding were observed in the cancer; in Group C, there was no ulcer in the cancer (Figure 1). HE staining showed: in Group A, mild infiltration of inflammatory cells and hyperplasia of fibroblasts were observed in the cancer; in Group B, the infiltration of inflammatory cells was evident, and hyperplasia of fibroblasts was every obvious, accompanied by dilation of vasculatures; in Group C, only a few inflammatory cells infiltrated in the cancer (Figure 2).
Figure 1: Macroscopic observation of the cancer on day 20. A: buccal cancer rabbits with infectious inflammation received intramuscular injection of procaine and penicillin as well as intragastrical injection of tinidazole for consecutive 3 days; B: buccal cancer rabbits with infectious inflammation received intragastrical and intramuscular injection of normal saline for 3 days; C: buccal cancer rabbits without infection received intragastrical and intramuscular injection of normal saline for 3 days.
Figure 2: Microscopic observation of the cancer on day 20 (HE staining; X200). A: buccal cancer rabbits with infectious inflammation received intramuscular injection of procaine and penicillin as well as intragastrical injection of tinidazole for consecutive 3 days; B: buccal cancer rabbits with infectious inflammation received intragastrical and intramuscular injection of normal saline for 3 days; C: buccal cancer rabbits without infection received intragastrical and intramuscular injection of normal saline for 3 days.
Morphology of DCs under a light microscope
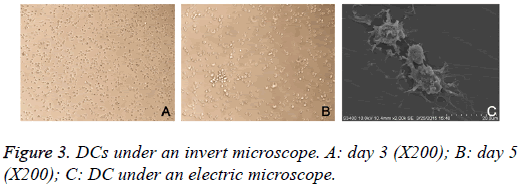
Cells were observed under an invert microscope. On day 3, a majority of cells were adherent to the wall and became oval or round. Burr-like protrusions were found in some cells. On day 5, cell volume increased, suspended or semi-suspended growth was found, some cells aggregated to form clusters, cells became star-like, spindle-shaped or irregular, and thorn-like projections of different sizes, numbers and shapes were observed in these cells. After collection of suspended cells, a few cells were still adherent to the wall (Figure 3). Under an electronic microscope, DCs became irregular in shape on day 6 and had rough surface, and there were a large amount of folds and dendritic-like protrusions in these cells (Figure 3).
HLA-DR and CD86 expression on DCs from VX2 cancer
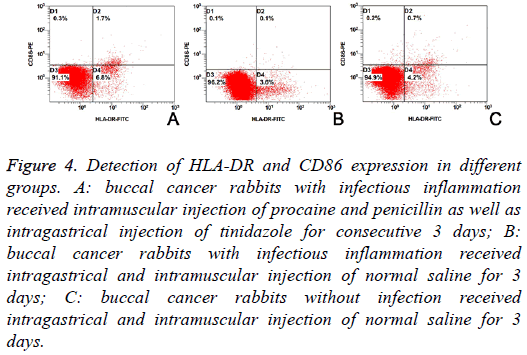
The HLA-DR and CD86 expression on DCs from VX2 cancer is shown in Table 1. Results showed the proportions of HLADR positive cells, CD86 positive cells and HLA-DR+CD86 positive cells were significantly different among three groups (F=216.658, 49.068 and 125.040; P<0.01). LSD-t showed proportions of HLA-DR positive cells, CD86 positive cells and HLA-DR+CD86 positive cells were the highest in Group A and the lowest in Group B (Figure 4 and Table 1).
| Group | HLA-DR | CD86 | HLA-DR+CD86 |
|---|---|---|---|
| A (n=6) | 9.77 ± 0.64#,☆ | 2.53 ± 0.47#,☆ | 2.20 ± 0.37#,☆ |
| B (n=6) | 3.56 ± 0.12▲ | 0.21 ± 0.04▲ | 0.07 ± 0.01▲ |
| C (n=5) | 5.02 ± 0.42 | 1.35 ± 0.57 | 0.75 ± 0.14 |
Table 1: Proportions of HLA-DR and/or CD86 positive cells in different groups.
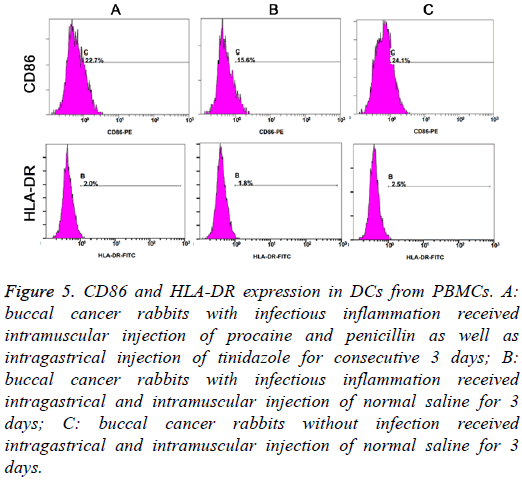
Figure 4: Detection of HLA-DR and CD86 expression in different groups. A: buccal cancer rabbits with infectious inflammation received intramuscular injection of procaine and penicillin as well as intragastrical injection of tinidazole for consecutive 3 days; B: buccal cancer rabbits with infectious inflammation received intragastrical and intramuscular injection of normal saline for 3 days; C: buccal cancer rabbits without infection received intragastrical and intramuscular injection of normal saline for 3 days.
HLA-DR and CD86 expression on DCs from PBMCs
On day 6, the proportions of HLA-DR positive cells, CD86 positive cells and HLA-DR+CD86 positive cells were markedly different among three groups (F=30.033, 40.664 and 18.444, respectively; P<0.01). LSD-t test indicated that the proportions of HLA-DR and/or CD86 positive cells were the highest in Group C and lowest in Group B (P<0.05) (Table 2 and Figure 5).
| Group | CD86 | HLA-DR |
|---|---|---|
| A (n=6) | 21.17 ± 2.30*,# | 2.13 ± 0.30*,# |
| B (n=6) | 13.95 ± 2.05*,☆ | 1.57 ± 0.38*,☆ |
| C (n=6) | 25.07 ± 1.84#,☆ | 2.60 ± 0.18#,☆ |
Table 2: Proportion of CD86 and/or HLA-DR positive cells in different groups
Figure 5: CD86 and HLA-DR expression in DCs from PBMCs. A: buccal cancer rabbits with infectious inflammation received intramuscular injection of procaine and penicillin as well as intragastrical injection of tinidazole for consecutive 3 days; B: buccal cancer rabbits with infectious inflammation received intragastrical and intramuscular injection of normal saline for 3 days; C: buccal cancer rabbits without infection received intragastrical and intramuscular injection of normal saline for 3 days.
Discussion
The oral and maxillofacial malignancies have a high incidence worldwide and more than 80% of them are OSCC [1]. Since mid-20 century, numerous studies have been conducted to investigate the relationship between inflammation and malignancies. Virchow, a pathologist in Germany, found inflammatory cells in cancers and for the first time proposed the hypothesis that inflammation is closely related to the pathogenesis of malignancies. Later, increasing studies confirm that inflammation is closely related to the occurrence, development and metastasis of malignancies [3]. In cancers, the inflammatory microenvironment mediates several signaling pathways via the pro-inflammatory cells and mediators, which forms a protective barrier to counteract the human immune defense, leading to the deterioration of cancers [4].
In the complicated microenvironment of the cancer, there are some immune surveillance cells and immune effector cells. These cells complicate the inflammatory microenvironment via their specific activities and differentiation [5]. DCs were identified by Steinman in 1973 and named due to the presence of dendrites in the mature cells. DCs have been the most potent Antigen Presenting Cells (APC) in the body, and its antigen presenting capability is stronger than that of any other APCs. DCs function mainly to up-take, process and manage antigens and present antigens to primitive T cells [6]. They are unique APCs that can activate primitive T cells. Thus, the DCs dysfunction may cause the whole immune dysfunction.
The functional status of DCs is closely related to the prognosis of malignancies. A variety of studies reveal that the DCs reduce, and the maturation and function of DCs are significantly compromised in the cancer microenvironment [7]. In the inflammatory microenvironment of the cancer, there is a large amount of pro-inflammatory cells and mediators, which may deteriorate this inhibition, and DCs usually fail to present antigens and activate T cells, and thus the recognition and killing of cancer cells are reduced, leading to the immune escape [8].
In cancers, there are a lot of DCs of different phenotypes and functions. DCs have been the most potent APCs and can be divided into immature DCs (imDCs) and mature DCs (mDCs) according to their ability to stimulate the proliferation of T cells. Under normal condition, the majority of DCs is immature and has the potent capability to take in, process and manage antigens. These cells have a low expression of MHC II molecules (including HLA-DR), costimulatory molecules (including CD86) and adhesion molecules, and the antigen presenting capability and their immune response are also very weak [9]. mDCs may not only present the first signal to T cells (propeptide-MHC complex), but provide the second signal (high expression of costimulatory molecules CD80, CD86 and CD40) for the complete activation of T cells. Thus, DCs are professional APCs that can directly activate primitive T cells. As a special group of cells, DCs play crucial roles in the activation and maintenance of anti-tumor immunity. In addition, the capability of DCs to present cancer antigens is lost or compromised in the inflammatory microenvironment of the cancer [10]. In the interaction between local inflammation and cancer cells, the expression of some pro-inflammatory cytokines is up-regulated (such as Cyclooxygenase-2 (COX-2), Vascular Endothelial Growth Factor (VEGF), Interleukin (IL)-1β, IL-6, IL-10, IL-8, IL-lα, and Transforming Growth Factor (TGF)), and these cytokines may affect the function of DCs and/or induce the apoptosis of DCs [11]. Of these cytokines, IL-1β and IL-6 are important pro-inflammatory cytokines and may stimulate the production of IL-10 by cancer cells to inhibit the chemotactic activity of DCs to antigens [12]. IL-10 can directly inhibit the expressions of MHC molecules and costimulatory molecules on DCs and block the cancer antigen presenting of DCs to T cells. Thus, the stimulating signals cannot be transmitted into T cells, and the T cells are in a non-responsive state, resulting in immune escape. In a variety of cancer patients, the expression of IL-1β, IL-6 and IL-10 increases significantly [13]. IL-6 may induce the VEGF expression in cancer cells, leading to immune suppression [14]. VEGF is an immunosuppressive factor and can inhibit the differentiation and maturation of DCs, resulting in immune escapes [15]. OSCC has a high expression of IL-1β and IL-6, which may promote VEGF release. In the cancer microenvironment, VEGF is crucial for the functional inhibition of DCs [16]. In addition, VEGF has a potent capability to inhibit the differentiation of CD34+hematopoietic stem cells, causing the immune escapes of cancer cells. This may be ascribed to the VEGF induced inhibition of NF-κB, which further blocks the function of DCs as shown in previous studies that the development and differentiation of DCs are closely related to the NF-κB. VEGF may bind to the type 1 VEGF receptor on HPC to block the separation of NF-κB from its inhibitor I-κB, which affects the NF-κB activity and inhibits the differentiation of hematopoietic precursor stem cells into DCs.
The cancer could be as large as 1.5-2.0 cm in diameter at 14 days after inoculation when the cancer cell growth is active and the necrotic tissues are small. The cancer grows and becomes larger over time. On day 21, the cancer becomes ulcerous, liquefaction necrosis may be observed in the cancer, and the survival time of cancer bearing rabbits is generally 40 days. Thus, in the present study, local inflammation was induced at 14 days after inoculation. Anti-infectious therapy was initiated on day 17 and continued for consecutive 3 days. On day 20, the cancer was collected for further examinations, which avoids the influence of cancer necrosis on our results.
The differentiation of PBMCs into DCs was evaluated under an invert microscope, and results showed, on day 3, a majority of cells were adherent to the wall, some cells showed suspended growth, cells were oval or round, and short, burrlike protrusions were observed in some cells; on day 5, some DCs showed suspended or semi-suspended growth, some DCs aggregated to form clusters, cells were star-like, spindle-shaped or irregular, and there were some thorn-like projections of different sizes and numbers were found on some cells. Under an electric microscope, DCs showed irregular shape, rough surface, a large amount of folds and dendritic-like protrusions of different sizes. These were characteristics of DCs.
The supernatant was collected from cancer tissues to mimic the cancer microenvironment and used to induce the differentiation of PBMCs into DCs. Then, flow cytometry was performed to detect the expression of CD86 and HLA-DR on cells. Results showed the expressions of CD86 and HLA-DR was the highest in Group C and the lowest in Group B; the SI was the highest in Group C and the lowest in Group B. This suggests that the inflammation in Group A and Group B inhibit the maturation and function of DCs, especially in Group B, and the expression of CD86 and HLA-DR and SI reduced significantly when compared with Group A. These findings indicate that the maturation, differentiation and function of DCs are significantly inhibited in the inflammatory microenvironment of cancer, and anti-infectious therapy may attenuate local inflammation, reduce the release of pro-inflammatory cytokines and then improve the maturation and function of DCs in this environment. In the presence of local inflammation, the expression of HLADR and CD86 in Group B was significantly lower than in Group C; after anti-infectious therapy for 3 days, the expression of HLADR and CD86 in Group A was markedly higher than in Group B and Group C. Thus, we speculate that local inflammation may inhibit the expression of functional molecules of DCs, and reduce the mature DCs. Antibiotics may kill the bacteria and then reduce the stimulation of inflammation, which is accompanied by the significant increase in the expression of HLADR and CD86, suggesting the increase in the number of mature DCs. Thus, anti-infectious therapy may increase DCs function. Taken together, in the infection induced inflammatory environment of OSCC, a large amount of pro-inflammatory cytokines may interact with each other, causing the disordered regulation of cytokine network, which further affects the expression of functional molecules on DCs and blocks the cancer antigen presenting. This may be one of mechanisms of immune escapes of cancer cells. However, the mechanisms by which the infectious inflammation inhibits the maturation and functions of DCs are still poorly understood. On the basis of our findings, we postulate that: 1) to maintain oral health, avoid local infection and use appropriate antibiotics at a proper dose to control local inflammation are helpful for the differentiation, maturation and function of DCs and reduce the invasion and metastasis of OSCC cells. 2) Mature DCs induced in vitro or DC vaccine may be used to treat oral cancer via improving the immunity of the host. This has been used in the study of Ruben et al. [17] in which the investigators administered DC vaccine in cancer patients and the survival time was significantly prolonged.
Acknowledgements
The study was supported by Science and Technology Project of Huagang District of Zunyi City (ZHKHSZ (2015) 09), Science and Technology Planning Project of Zunyi City (ZSKHSZ (2013) 32), and Returnee Scientific Research Fund of Ministry of Education (JWSL (2011) 508).
References
- Eich HT, Loschcke M, Scheer M, Kocher M, Bongartz R, Wacker S, Zoller JE, Muller RP. Neoadjuvantradiochemotherapy and radical resection for advanced squamous cell carcinoma of the oral cavity. Outcome of 134 patients. StrahlentherOnkol 2008; 184: 23-29.
- King M, Chatelain K, Farris D, Jensen D, Pickup J, Swapp A, OMalley S, Kingsley K. Oral squamous cell carcinoma proliferative phenotype is modulated by proanthocyanidins: a potential prevention and treatment alternative for oral cancer. BMC Complement Altern Med 2007; 7: 22.
- Mantovani A. Cancer: Inflaming metastasis. Nature 2009; 457: 36-37.
- Schwartsburd PM. Chronic inflammation as inductor of pro-cancer microenvironment: pathogenesis of dysregulated feedback control. Cancer Metastasis Rev 2003; 22: 95-102.
- Lin A, Schildknecht A, Nguyen LT, Ohashi PS. Dendritic cells integrate signals from the tumor microenvironment to modulate immunity and tumor growth. ImmunolLett 2010; 127: 77-84.
- Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity 2014; 40: 642-656.
- Fainaru O, Almog N, Yung CW, Nakai K, Montoya-Zavala M, Abdollahi A, DAmato R, Ingber DE. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. Faseb J 2010; 24: 1411-1418.
- Li N, Grivennikov SI, Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell 2011; 19: 429-431.
- Inaba K, Turley S, Iyoda T, Yamaide F, Shimoyama S, Reis e Sousa C, Germain RN, Mellman I, Steinman RM. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med 2000; 191: 927-936.
- Palombo F, Focaccetti C, Barnaba V. Therapeutic implications of immunogenic cell death in human cancer. Front Immunol 2014; 4: 503.
- Forget P, Coulie PG, Retsky M, Demicheli R, Machiels JP. Is there a rationale for an anesthesiologists role against cancer recurrence? ActaAnaesthesiolBelg 2013; 64: 15-24.
- Bonomi M, Patsias A, Posner M, Sikora A. The role of inflammation in head and neck cancer. AdvExp Med Biol 2014; 816: 107-127.
- Zhang M, Zhou S, Zhang L, Ye W, Wen Q. Role of cancer-related inflammation in esophageal cancer. Crit Rev Eukaryot Gene Expr 2013; 23: 27-35.
- Middleton K, Jones J, Lwin Z, Coward JI. Interleukin-6: an angiogenic target in solid tumours. Crit Rev OncolHematol 2014; 89: 129-139.
- Oh H, Takagi H, Otani A, Koyama S, Kemmochi S, Uemura A, Honda Y. Selective induction of neuropilin-1 by vascular endothelial growth factor (VEGF): a mechanism contributing to VEGF-induced angiogenesis. ProcNatlAcadSci USA 2002; 99: 383-388.
- Burkly L, Hession C, Ogata L, Reilly C, Marconi LA. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature 1995; 373: 531-536.
- Ruben JM, van den Ancker W, Bontkes HJ, Westers TM, Hooijberg E, Ossenkoppele GJ, de Gruijl TD, van de Loosdrecht AA. Apoptotic blebs from leukemic cells as a preferred source of tumor-associated antigen for dendritic cell-based vaccines. Cancer ImmunolImmunother 2014; 63: 335-345.