ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 4
Inhibitory effects of the active constituents of ginseng stems and leaves on lung cancer NCI-H1650 cells
Mengli Zheng, Naikang Zhou*, Lianjun Ma, Wenbo Jin
Department of General Thoracic Surgery, The 309th Hospital of Chinese PLA, Beijing 100091, China
- *Corresponding Author:
- Naikang Zhou
Department of General Thoracic Surgery
The 309th Hospital of Chinese PLA
Beijing 100091,China
Accepted : August 08 2015
This research is aimed to isolate the active constituents of Ginseng stems and leaves and study their anti-cancer activities. Preparative TLC, spectral data analysis and MTT assay are used to isolate constituents and study anti-cancer activities. Six compounds are isolated from Ginseng stems and leaves, namely 20(S)-ginsenoside Rh1, ginsenoside F3, daucosterol, β-sitosterol, ginsenoside Rd2 and 20(R)-ginsenoside Rg2. Inhibitory effect of ginsenoside Rg2 on growth of NCI-H1650 cells is detected by MTT and Annexin-V-PI double staining assays. The results show that different concentrations of ginsenoside Rg2 test solutions can inhibit the growth of NCIH1650 cells to varying degrees, and the effect presents evident dose-response and time-effect relationships, that is, the longer the action time, the stronger the inhibitory effect. It can be concluded that the active constituents in Ginseng stems and leaves have significant anti-cancer activity.
Keywords
Ginseng stem and leaf; Annexin-V-PI; NCI-H1650 cell; ginsenoside Rg2
Introduction
Ginseng is the dried root of perennial herb Panax ginseng C. A. Meyer in the Araliaceae family, which is a characteristic traditional Chinese medicine; it is mainly produced in China's three northeastern provinces, especially in Jilin, which has a long history of medicinal use [1,2]. Modern research has shown that Ginseng contains saponins, polysaccharides, amino acids, vitamins, flavonoids, fatty acids, volatile oils, trace elements, etc. [3]. Its main active constituents are ginsenosides. Currently, at least 40 types of ginsenoside monomers have been isolated from ginseng plants.
Modern pharmacological studies have shown that ginseng can improve cardiac contractility and frequency, stimulate the heart, and accelerate blood flow; has a good protective effect on ischemia-induced myocardial injury; has antishock and anti-stress effects; can excite the central nervous system, and protect ischemic brain cells [4,5]. What is of particular importance is that ginsenosides have good anti-tumor activities [6-8], which can complement the drawbacks of surgery, radiotherapy and chemotherapy, and prevent side effects, and thereby allow reduced toxicity and enhanced efficacy when used in combination with chemotherapeutics, provide effects such as improved immunity, alleviate the suffering of cancer patients, improve their quality of life, and prolong their survival time.
To better protect the forests, and achieve sustainable development of ginseng use value, Chinese government has issued a series of policies to strictly control the artificial cultivation of ginseng. Strict control of ginseng cultivation area has greatly affected the widespread use of ginseng, so the search for new medicinal parts to expand the usable range of ginseng has become an important topic. This paper extracts and isolates active constituents in Ginseng stems and leaves, and studies their anti-cancer activities.
Materials & Methods
Instruments and reagents
Melting point apparatus (uncorrected, Shimadzu, Japan); Bruker FT-IR infrared spectrometer; BrukerAV superconducting NMR spectrometer; 100-300 mesh column chromatography silica gel (Qingdao Haiyang Chemical Co., Ltd.); Sephadex LH-20 column chromatography gel (Pharmacia); MTT and DMSO (AMRESCO); PI (Sigma); SW-CJ-2F super clean bench (Suzhou Purification Equipment Factory); micromill.
Drugs and cells
Ginseng stems and leaves; 7-8 years old, which were harvested in summer and autumn and air-dried, were collected from ginseng farmers in Fusong, Jilin, and identified as Araliaceae plant ginseng. Ginsenoside Rg2 was self-prepared, and NCI-H1650 cell line was purchased from Shanghai FuXiang Biotechnology Co., Ltd.
Extraction and isolation of compounds
5 kg of dried ginseng leaves were crushed, and extracted by boiling with 8-fold amount of water, 3 times for 2 h each. Then, the filtrates were combined, concentrated, and eluted through AB-8 macroporous resin (extract amount: resin amount = 1:8) with 85% ethanol. After removal of ethanol, it was extracted successively with ethyl acetate and n-butanol. Then, the extracts were combined, and extractants were evaporated to give ethyl acetate fraction and n-butanol fraction. Next, ethyl acetate fraction was subjected to silica gel column chromatography, and gradient eluted with chloroform-ethanol-water, similar eluents were then combined, and further isolated by preparative TLC, repeated silica gel column chromatography and ODS column chromatography to give compounds 1-3. N-butanol fraction was subjected to silica gel column chromatography, gradient eluted with ethyl acetate-ethanol-water, and then isolated by preparative TLC to give compounds 4-6.
Cell culturing
NCI-H1650 cells were cultured routinely in 10% FBScontaining RPMI 1640 medium, and incubated at a 37℃, 5% CO2 incubator. The medium was replaced once every 3 day. Logarithmic phase cells were used for experiment.
MTT assay
Logarithmic phase NCI-H1650 cells were trypsinized routinely, prepared into a 1×105/mL cell suspension, seeded in 96-well plates, and then added with different concentrations (20 umol/L, 40 umol/L, 60 umol/L and 80 umol/L) of ginsenoside Rg2 test solutions. Control group was added with an equivalent volume of PBS. After culturing for 24 h, 36 h and 48 h, each well was added with 20 μL of 5 mg/mL MTT solution, and cultured for an additional 4 h. Next, the supernatant was discarded, and 100 μL of DMSO was added to each well. After the plates were shaken, A550 of each well was measured with ELISA reader. Finally, NCI-H1650 cell inhibition rates of different concentrations of ginsenoside Rg2 test solutions were calculated.
Inhibition rate = (average A550 of control group - average A550 of treatment group) / average A550 of control group × 100%
Detection of apoptosis cycle by Annexin-V-PI double staining assay
NCI-H1650 cells with a concentration of 5×105/ml were seeded in 96-well plates, and treated with different concentrations (40 umol/L and 80 umol/L) of ginsenoside Rg2 test solutions, as well as an equivalent volume of PBS. After culturing for 24 h, cells in each group were collected separately. Each group consisted of three wells, each of which was added with 5 μL of Annexin V/FITC, mixed well, then added with 5 μL of PI, and reacted in the dark at room temperature for 15 min, followed by analysis on flow cytometer.
Statistical processing
Data were processed using SPSS 13.0 statistical software,
and measurement data were expressed as  ±s.
Comparison among groups was performed by t test, and
P<0.05 was considered statistically significant.
±s.
Comparison among groups was performed by t test, and
P<0.05 was considered statistically significant.
Results
Structure elucidation of compounds
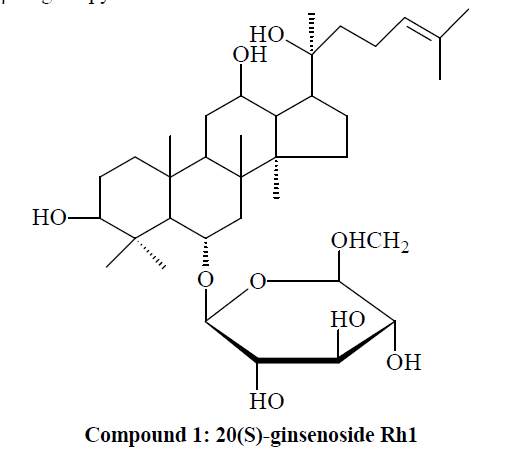
Compound 1: White powder. 1H-NMR (C5D5N, J=Hz) δ(ppm): 0.76, 0.98, 1.14, 1.27, 1.44, 1.53, 1.55, 1.91 (3H, each, alls, H-18, 19, 30, 29, 28, 26, 27, 21); 4.93 (1H, d, J=7.8, 6-O-glc-H-1'); 13C-NMR (75 MHz, DMSO-d6) δ: 39.8 (C-1), 28.9 (C-2), 79.1 (C-3), 41.2 (C-4), 62.2 (C-5), 19.0 (C-6), 45.9 (C-7), 41.1 (C-8), 50.0 (C-9), 41.1 (C-10), 32.7 (C-11), 72.9 (C-12), 48.9 (C-13), 52.3 (C-14), 32.1 (C-15), 27.7 (C-16), 55.5 (C-17), 18.9 (C-18), 18.1 (C-19), 73.5 (C-20), 27.8 (C-21), 35.6 (C-22), 23.9 (C-23), 127.6 (C-24), 134.1 (C-25), 24.6 (C-26), 18.1 (C-27), 32.5 (C- 28), 16.9 (C-29), 16.6 (C-30). The above data were basically consistent with those in the literature [9], so compound 1 was identified as 20(S)-ginsenoside Rh1, i.e. dammar-24-alkenyl-3β,6α,12β,20(S)-tetrahydroxy-20-O- β-D-glucopyranoside.
Compound 2: White powder. 1H-NMR (C5D5N, J=Hz) δ (ppm): 0.83, 0.86, 0.95, 1.38, 1.53, 1.55, 1.56, 1.89 (3H, each, alls, H-18, 19, 30, 29, 28, 26, 27, 21), 5.16 (1H, d, J=7.5, 20-O-glc-H-1'); 13C-NMR (75 MHz, DMSO-d6) δ: 39.9 (C-1), 28.8 (C-2), 79.2 (C-3), 41.9 (C-4), 62.1 (C-5), 69.1 (C-6), 46.9 (C-7), 41.2 (C-8), 50.9 (C-9), 40.1 (C-10), 32.1 (C-11), 69.9 (C-12), 49.9 (C-13), 52.5 (C-14), 31.3 (C-15), 27.9 (C-16), 52.1 (C-17), 17.9 (C-18), 17.6 (C-19), 84.1 (C-20), 22.9 (C-21), 36.6 (C-22), 24.6 (C-23), 127.6 (C-24), 132.4 (C-25), 24.6 (C-26), 17.9 (C-27), 31.8 (C- 28), 17.2 (C-29), 16.9 (C-30). The above data were basically consistent with those in the literature [10], so compound 2 was identified as ginsenoside F3, i.e. dammar-24-alkenyl-3β,6α,12β,20(S)-tetraol-20-O-β-Dglucopyranosyl- α-L-arabinopyranoside.
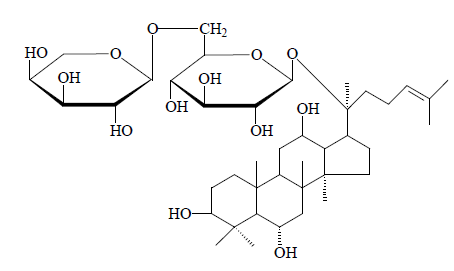
Compound 2: Ginsenoside F3
Compound 3: White powder (methanol). Lieberman- Burchard reaction and Molisc test: positive. TLC with three different solvents revealed that the Rf values of three solvents were all consistent with the reference; besides, melting points did not drop after mixing. So compounds 3 was identified as daucosterol.
Compound 4: White needle crystals (acetone), mp 140~142℃. Lieberman-Burchard reaction: positive. TLC on the same plate with β-sitosterol reference revealed that the Rf values were all consistent under three different solvent conditions; besides, melting points did not drop after mixing. So compounds 4 was identified as β- sitosterol.
Compound 5: White powder. 1H-NMR (C5D5N, J=Hz) δ(ppm): 0.66, 0.82, 0.82, 0.85, 1.22, 1.51, 1.54, 1.58 (3H, each, alls, H-18, 19, 30, 29, 28, 26, 27, 21), 5.11 (1H, d J=7.5, 20-O-glc-H-1'), 4.91 (1H, d, J=7.5, 3-O-glc-H-1'); 13C-NMR (75 MHz, DMSO-d6) δ: 39.8 (C-1), 27.9 (C-2), 89.6 (C-3), 38.1 (C-4), 58.1 (C-5), 18.7 (C-6), 35.5 (C-7), 41.3 (C-8), 48.1 (C-9), 37.9 (C-10), 31.4 (C-11), 71.3 (C- 12), 50.6 (C-13), 52.5 (C-14), 30.6 (C-15), 26.7 (C-16), 51.7 (C-17), 16.4 (C-18), 16.5 (C-19), 83.7 (C-20), 22.2 (C-21), 36.3 (C-22), 23.2 (C-23), 125.3 (C-24), 131.7 (C- 25), 25.7 (C-26), 17.3 (C-27), 27.1 (C-28), 17.2 (C-29),
17.9 (C-30). Upon comprehensive analysis, spectral data of compound 5 were found basically consistent with the literature [11], so compound 5 was identified as ginsenoside Rd2, i.e. dammar-24(25)-alkenyl- 3β,12β,20(S)-triol-20-O-α-L-arabinopyranosyl(1→6)-β- D-glucopyranosyl-3-O-β-D- glucoside.
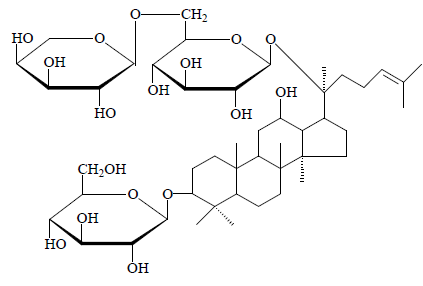
Compound 5: Ginsenoside Rd2
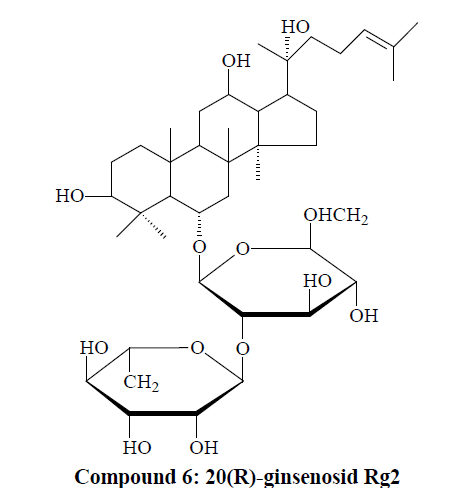
Compound 6: White powder. 1H-NMR (C5D5N, J=Hz) δ(ppm): 0.95, 0.94, 1.16, 1.26, 1.28, 1.61, 1.66, 1.95, 1.94 (3H, each, alls, H-18, 19, 30, 29, 28, 26, 27, 21), 5.17 (1H, d, J=6.5, 6-O-glc-H-1'); 13C-NMR (75 MHz, DMSO-d6) δ: 39.7 (C-1), 27.9 (C-2), 78.7 (C-3), 41.2 (C-4), 62.2 (C-5), 75.6 (C-6), 47.2 (C-7), 41.1 (C-8), 51.6 (C-9), 40.6 (C-10), 33.5 (C-11), 72.3 (C-12), 48.4 (C-13), 52.3 (C-14), 31.4 (C-15), 26.7 (C-16), 50.1 (C-17), 17.5 (C-18), 17.9 (C-19), 73.1 (C-20), 22.4 (C-21), 43.2 (C-22), 22.1 (C-23), 125.4 (C-24), 131.4 (C-25), 25.3 (C-26), 16.4 (C-27), 31.5 (C- 28), 17.7 (C-29), 17.9 (C-30). Upon comprehensive analysis, spectral data of compound 6 were found basically consistent with the literature [12], so compound 6 was identified as 20(R)-ginsenosid Rg2.
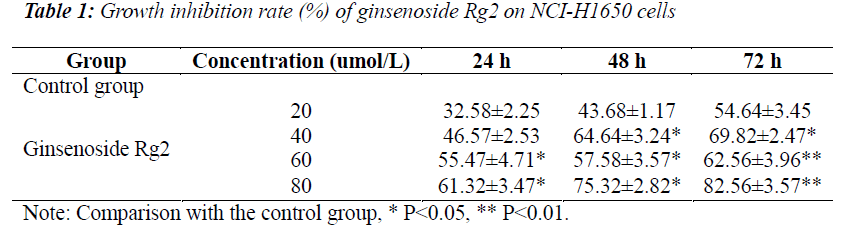
Determination of NCI-H1650 cell inhibitory effect by MTT assay
Compared with the control group, different concentrations of ginsenoside Rg2 test solutions could all inhibit the growth of NCI-H1650 cells to varying degrees, and their effect presented evident dose-response and time-effect relationships, that was, the longer the action time, the Inhibitory effects of ginseng stems and leaves on lung cancer NCI-H1650 cells. stronger the inhibitory effec
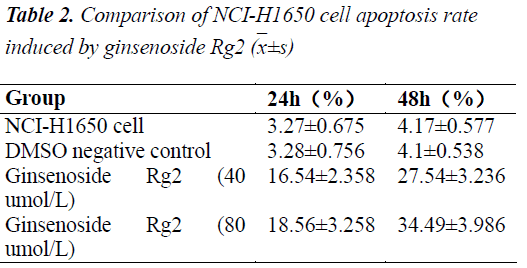
Apoptosis promoting effect of ginsenoside Rg2 on NCIH1650 cells
Detection results showed that after acting on the NCIH1650 cells for 24 h, test concentrations of ginsenoside Rg2 could all evidently promote apoptosis. Apoptosis rates, whether early or late, were significantly higher for the ginsenoside Rg2 groups than the normal group (P<0.01); besides, high concentration treatment group had early apoptosis rate significantly higher than the low concentration treatment group (P<0.05), indicating that ginsenoside Rg2 had an apoptosis promoting effect on NCI-H1650 cells, which was somewhat does dependent.
Discussion
Ginseng root has been used as a traditional Chinese medicine for a long time. Before large-scale industrial production, the need for ginseng as a disease-curing and life-saving product had been basically met by harvesting wild ginseng. With the continuous improvement of people's living standards and growing awareness of health care, the demands for ginseng and other traditional medicines have been increasing, and ginseng production has also developed gradually from wild harvesting to artificial cultivation. However, along with the soil and water loss and gradual lack of soil components, cultivation of ginseng has been increasingly restricted, leading to extreme imbalance between supply and demand. Therefore, the development of new medicinal parts of ginseng and the search for new alternatives have been hot research topics. In this paper, six compounds are isolated from ginseng stems and leaves, namely 20(S)- ginsenoside Rh1, ginsenoside F3, daucosterol, β-sitosterol ginsenoside Rd2 and 20(R)-ginsenoside Rg2.
Lung cancer is one disease that has now become the malignancy with the highest incidence and mortality, worldwide. Its pathogenesis is unclear yet, but lies in the deregulation of cell proliferation and apoptosis. In recent years, research of the anticancer mechanism of traditional Chinese medicine has made great progress, and some traditional Chinese preparations such as Brucea javanica oil injection, paclitaxel injection and cantharidin-related preparations have been used in the treatment of tumors. Active search for highly effective, low toxic cancer prevention and treatment drugs has become one of the urgent tasks faced by China and the world today.
There have been many reports on the anti-tumor activity of ginsenosides in recent years. Experimental studies have shown that ginsenosides can inhibit the production of tumor cells through a variety of channels. The first channel is a direct effect on cancer cells, where tumor growth is inhibited by induction of apoptosis [13]. By inducing apoptosis, tumor cell death is artificially initiated to treat or eliminate tumors, and restore the dynamic equilibrium between cell proliferation and apoptosis. The second channel is induction of tumor cell differentiation [14], and reversal of tumor cell differentiation. The third channel is inhibition of tumor invasion and metastasis [15]. Tumor angiogenesis is the basis of tumor growth and metastasis. Ginseng constituents such as ginsenosides Rb2 and Rg3 can destroy or inhibit the angiogenesis, thereby inhibiting tumor growth and metastasis. The fourth channel is enhancement of immune function [16]. Ginsenosides can enhance immune activity, regulate cytokine production, and mediate multiple immune responses, and thereby effectively inhibit tumor cells.
In this paper, the inhibitory effect of ginsenoside Rg2 on growth of NCI-H1650 cells is detected by MTT and Annexin-V-PI double staining assays. The results show that different concentrations of ginsenoside Rg2 test solutions can all inhibit the growth of NCI-H1650 cells to varying degrees, and the effect presents marked doseresponse and time-effect relationships, that is, the longer the action time, the stronger the inhibitory effect.
Ginsenoside Rg2 can well promote the apoptosis of NCIH1650 cells, which shows a certain dose-response relationship. Its mechanisms of action will be explored progressively in subsequent studies.
References
- Chinese Pharmacopoeia Commission.Pharmacopoeia of the People's Republic of China (Vol.I). Chemical Industry Press 2010: 131-132.
- Gu GG. Sheng Nong's Herbal Classic. Beijing: People's Medical Publishing House 1956: 2S.
- Xun XH, Zhou JL. Progress of research on ginsenosides. China Journal of Modern Medicine 2003; 13: 43-44.
- Qin XC, Wei DY, Guo XW. Effects of ginsenosidesRb on free radical metabolism in rats with acute myocardial infarction. Jilin Journal of Traditional Chinese Medicine 2002; 22: 51-55.
- Coleman CL, Hebert JH, Reddy P. The effeets of Panaxginseng on quality of 1ife. Journal of Clinieal Pharmaey and TheraPeuties 2003; 28: 5-15.
- Chen DL, Liu YG, Wang YP, Chen TM, Zheng M, Jiang R. Effects of Ginseng polysaccharide on proliferation and differentiation of K562 cells. Journal of Third Military Medical University 2005; 27: 517-520.
- Chang YS, Seo EK,Gyllenhaal C. Panax ginseng: A Role in CaneerTheraPyIntegr Cancer Ther 2003; 2: 13-33.
- Ham YM, Chun KH, Choi JS. SEK1-dependent JNK1 activation prolongs cell surmvaldunng GRh2- induced apoptosis. BiochemRiophps Res Commuu 2003; 304: 358-364.
- Song CC, Ma XY, Xu JD. Preparation of ginsenosides Rh1 and Rh2 with Panaxquinquefoliussaponins.Chinese Pharmaceutical Journal 1992; 27: 6-9.
- Dou DJ, Wen Y, Pei YP, Chen YJ, Ma ZZ. Study on trace constituents in ginseng leaves. China Journal of Chinese MateriaMedica 1997; 22: 35-38.
- DOU DQ, CHEN YJ, MENG ZY. Two minor saponins from leaves of Panax ginseng C.A. Meyer.Journal of Chinese Pharmaceutical Sciences 1996; 5: 195-199.
- Yang XW. Complete assignment of 1H and 13CMNR chemical shifts of 20(R)-ginsenoside and 20(S)- ginsenoside Rg2. Chinese Journal of Magnetic Resonance 2000; 17: 9-15.
- Kang KS, Kang BC, Lee BJ, Che JH, Li GX, TroskoJE, Lee YS. Preventive effect of epicatechin and ginsenoside Rb2 on the inhibition of gap junctionalintercellular communication by TPA and H2O2.Cancer Lett 2000; 152: 97-106.
- Luo JS, Gu LX, Xi HJ, Wei BJ, Liu XB, Qiu JW, Zhang PF, Shan HR. Cytotoxicity of GS and IL-2 activated PBMC on malignant glioma cells. Chinese Journal of Cancer Biotherapy 2000;7: 273-274.
- Tatsuka M, Maeda M, Ota T. Anticarcinogenic Effect and Enhancement of Metastatic Potential of BALB / c 3T3 Cells by Ginsenoside Rh2. Jpn J Cancer Res 2001; 92: 1184-1189.
- Wargovich MJ. Colon cancer chemoprevention with ginseng and other botanicals. J Korean Med Sci 2001; 16 Suppl: 81-86.