ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Paper - Biomedical Research (2022) Volume 33, Issue 6
Interferon-gamma released from natural killer cell and breast cancer progression.
Eunhae Um, Jae Il Kim*
Department of Surgery, Division of Breast Surgery, Inje University Ilsan Paik Hospital, Goyang, Republic of Korea
- Corresponding Author:
- Jae Il Kim
Department of Surgery
Division of Breast Surgery
Inje University Ilsan Paik Hospital
Goyang
Republic of Korea
Accepted date: August 11, 2022
Background: Natural Killer (NK) cells are lymphocytes that have a spontaneous cytotoxic activity to recognize and eliminate malignantly transformed cells through early defense against tumors. We aimed to investigate the association between NK cell activity and tumor characteristics, especially in TNM stage, in patients with breast cancer.
Methods: This was a single-center retrospective study. Newly diagnosed breast cancer patients from July 2015 to December 2019 were analyzed. Cytotoxic activity of NK cells was determined using the NK Vue-Kit® (ATgen, Sungnam, Korea) which estimated the level of Interferon-Gamma (IFN-γ) released from NK cells. We transformed IFN-γ levels into natural logs (lnIFN-γ) and analyzed them.
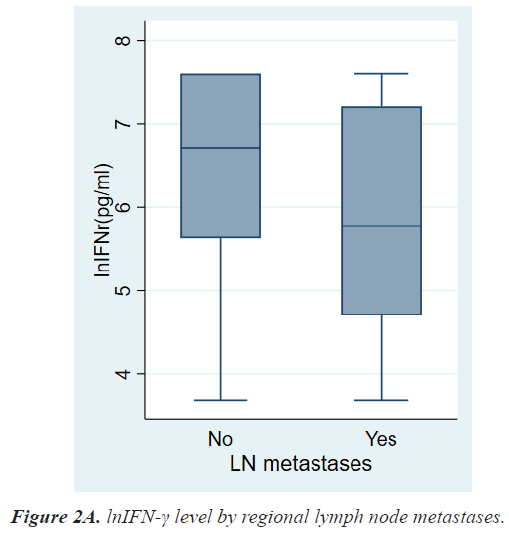
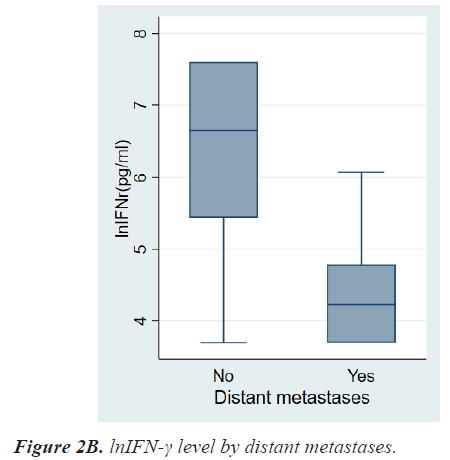
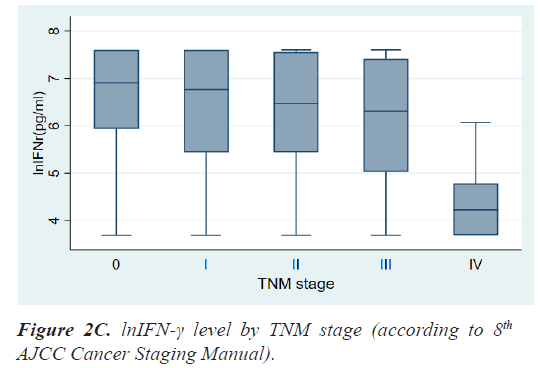
Results: The mean age at diagnosis was 56.3 ± 12.5 years. According to the TNM stage, the median level and range of lnIFN-γ in stage 0, 1, 2, 3, and 4 were 6.91(3.69-7.60) pg/ml, 6.76(3.69-7.60) pg/ml, 6.47(3.69-7.60) pg/ ml, 6.31(3.69-7.60) pg/ml, and 4.23(3.69-6.07) pg/ml, respectively and the P-value was 0.0013. Patients with LN metastases had a significantly lower level of lnIFN-γ than those without LN metastasis (5.78 (3.69-7.60) vs. 6.71 (3.69-7.60) pg/ml, P=0.0017). Patients with distant metastases had also a significantly lower level of lnIFN-γ than those without distant metastasis (4.23, 3.69-6.07 vs. 6.64, 3.69-7.60 pg/ml, P<0.0001).
Conclusion: Progressed breast cancer stage leads to a lower level of lnIFN-γ. It is assumed that IFN-γ released from NK cells decreases as cancer progresses. We suggest that the measurement of IFN-γ levels released from NK cells can be a useful method of surveillance for breast cancer patients.
Keywords
Interferon-gamma, Natural killer cell, Breast cancer.
Introduction
The immune system is an interactive defense network of cells and proteins which enable the host to recognize, repel, and eradicate pathogens and other foreign molecules. Thus, the immune system protects our body against infectious diseases and maintains system integrity.
There are two branches of immune systems in our body, innate immunity which is also known as the in-born immunity system, and adaptive immunity, also known as the acquired immune system. Innate immunity includes Natural Killer (NK) cells, skin, macrophages, granulocytes, and monocytes.
NK cells are a type of cytotoxic lymphocyte critical to the innate immune system and are classified as group 1 Innate Lymphoid Cells (ILCs) [1]. NK cells recognize and lyse malignant transformed and virus-infected cells through early defense against tumors and infections [2]. Without Major Histocompatibility Complex (MHC) restriction or prior sensitization, NK cells can eliminate target cells with malignant transformation or intracellular microbe infection [3]. NK cells are activated by the binding of NK cell-activating receptors with activating ligands and also activated by proinflammatory cytokines such as Interleukin (IL)-2, IL-12, IL-15, and IL-18 [3,4]. Activated NK cells directly lyses target cells by releasing perforin and various granzymes and also produce cytokines such as Interferon (IFN)-γ which is a major cytokine to exert immune responses against cancer cells and virus-infected cells.
Breast cancer is the most common malignancy in women around the world. Although mortality has been reduced due to emerging new treatment strategies, breast cancer is still the most common cause of death from malignancy. The molecular subtypes of breast cancer depend on the Hormone Receptor (HR) and Human Epidermal Growth Factor Receptor 2 (HER2) status. The importance of these biological factors increases as the therapeutic strategy depends more on the subtype than the severity of the disease. In the revised eighth edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, demonstrated the importance of biological factors by including a new prognostic staging system.
In the last few decades, numerous studies have been conducted on NK cells or immunotherapy and revealed evidence that immune response has a significant role in oncologic outcomes. Previously, few reports have been made on the relationship between NK cell Activity (NKA) and breast cancer. In this study, we aimed to investigate the association between NKA and tumor characteristics, especially in TNM stage, in patients with breast cancer.
Materials and Methods
Study population
This was a single-center retrospective study. Medical records of patients who were newly diagnosed with breast cancer, either ductal carcinoma in situ or invasive breast cancer, from July 2015 to December 2019 were collected. Patients of all stages, including de novo stage IV patients, were included. However, patients with a previous history of any malignancy, history of chemotherapy or radiotherapy, immuno compromised state, or infected state with any virus or bacteria were excluded. A chart review was performed to collect demographic data and tumor characteristics, such as invasion of basement membrane, histologic grade, tumor size, regional lymph node metastasis, distant metastasis, estrogen and progesterone receptor (ER and PgR) status, and HER2 status.
The breast cancer stage was classified from stage 0 to stage 4 according to the 8th AJCC Cancer Staging Manual. We adopted the breast cancer subtyping classification according to St. Gallen International Expert Consensus [5]: patients were categorized as luminal A (ER+ and/or PgR+, HER2- and Ki-67 low), luminal B (ER+ and/or PgR+, HER2- and Ki-67 high), luminal B-HER2 positive (ER and/or PgR+, HER2+, any Ki-67), HER2-positive (ER- and PgR- and HER2+) and triple-negative (ER- and PgR- and HER2-).
This study was approved by the institutional review board of ooo (IRB no. 2021-02-015). The need for informed consent was waived.
NK VueTM assay
Cytotoxic activity of NK cells was determined using the NK Vue-Kit® (ATgen, Sungnam, Korea) which estimated the level of IFN-γ released from NK cells. Whole blood was collected using BD Vacutainer® heparinN1 tubes after diagnosis of breast cancer. 1 ml of whole blood was incubated for 24 hrs, at 37°C, under 5% CO2 with the indicated dose of Promoca® and 1 ml of RPMI 1640 media. Cell-free supernatants were harvested, and IFN-γ levels were determined according to the manufacturer’s protocols. The results were reported as values between 40 to 2000 pg/ml. IFN-γ levels over 250 pg/ml were considered normal NKA.
Statistical analysis
IFN-γ levels were transformed into natural logs (lnIFN-γ) for analysis and used the Mann-Whitney U test or Kruskal- Wallis test as non-parametric statistical methods. A P-value of less than 0.05 was considered statistically significant. Statistical analyses were performed using STATA (version 16.0, StataCorp LP, College Station, TX, USA).
Results
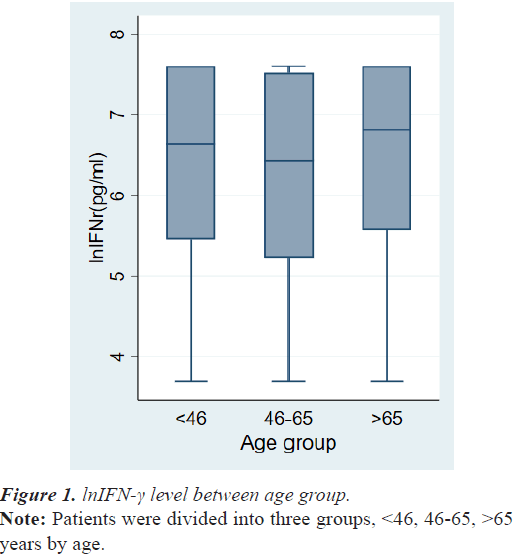
The demographic data and tumor characteristics of 268 breast cancer patients are summarized in Table 1. The mean age at diagnosis was 56.3 ± 12.5 years. The number of cases of TNM stage 0, stage 1, stage 2, stage 3 and stage 4 were 43 (16.0%), 81 (30.2%), 106 (39.6%), 29 (10.8%), and 9 (3.4%), respectively. There was no difference in lnIFN-γ levels among the patient’s age group (Figure 1).
| Variables | N | %, Mean(± SD) or Median(range) |
|---|---|---|
| Age | 268 | 56.3 ± 12.5 yrs |
| IFN-γ | 268 | 731.1(40.0-2000.0) pg/ml |
| lnIFN-γ | 268 | 6.59(3.69-7.60) pg/ml |
| Histologic grade 1 2 3 NA |
70 128 59 11 |
26.1% 47.8% 22.0% 4.1% |
| Invasion to BM Carcinoma in situ Invasive carcinoma |
43 225 |
16.0% 84.0% |
| Lymph node metastases No Yes |
188 80 |
70.1% 29.9% |
| Distant metastases No Yes |
259 9 |
96.6% 3.4% |
| TNM stage 0 I II III IV |
43 81 106 29 9 |
16.0% 30.2% 39.6% 10.8% 3.4% |
| HR status Positive Negative |
195 73 |
72.8% 27.2% |
| HER2 status Positive Negative |
81 187 |
30.2% 69.8% |
| Subtype Luminal A Luminal B Luminal_HER2 HER2 TNBC |
65 87 43 38 35 |
24.2% 32.5% 16.0% 14.2% 13.1% |
Table 1. Demographic data and tumor characteristics.
Table 2 shows the results of comparative analyses regarding lnIFN-γ by various tumor-related factors. The normal range of lnIFN-γ level is 5.52-7.60 pg/ml. Among various tumor-related factors, TNM stage, LN metastasis, and distant metastasis showed significant differences in lnIFN-γ levels (Figures 2a-2c). According to the TNM stage, the median level and range of lnIFN-γ in stage 0, 1, 2, 3, and 4 were 6.91(3.69-7.60) pg/ml, 6.76(3.69-7.60) pg/ml, 6.47(3.69-7.60) pg/ml, 6.31(3.69-7.60) pg/ml, and 4.23(3.69-6.07) pg/ml, respectively (P=0.0013). Patients with LN metastases had a significantly lower level of lnIFN-γ than those without LN metastasis (5.78, 3.69-7.60 vs. 6.71, 3.69-7.60 pg/ml, P=0.0017). Patients with distant metastases had also a significantly lower level of lnIFN-γ than those without distant metastasis (4.23, 3.69-6.07 vs. 6.64, 3.69-7.60pg/ml, P<0.0001).
| Variables | lnIFN-γ (pg/ml), median(range) | P-value |
|---|---|---|
| Age ≤ 45 46-65 >65 |
6.64(3.69-7.60) 6.43(3.69-7.60) 6.81(3.69-7.60) |
0.1889 |
| Histologic grade 1 2 3 |
6.87(3.69-7.60) 6.44(3.69-7.60) 6.75(3.69-7.60) |
0.1632 |
| Invasion to BM Carcinoma in situ Invasive carcinoma |
6.91(3.69-7.60) 6.51(3.69-7.60) |
0.0656 |
| Lymph node metastases No Yes |
6.71(3.69-7.60) 5.78(3.69-7.60) |
0.0017 |
| Distant metastases No Yes |
6.64(3.69-7.60) 4.23(3.69-6.07) |
<0.0001 |
| TNM stage 0 I II III IV |
6.91(3.69-7.60) 6.76(3.69-7.60) 6.47(3.69-7.60) 6.31(3.69-7.60) 4.23(3.69-6.07) |
0.0013 |
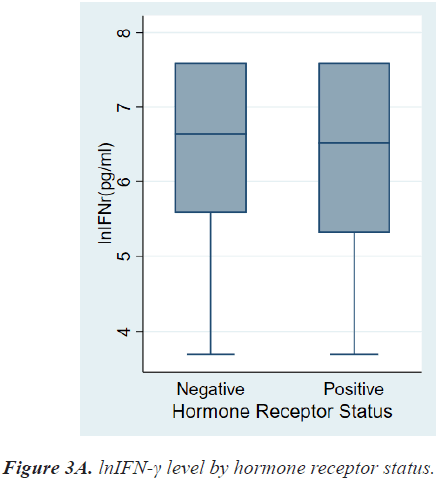
| HR status Positive Negative |
6.52(3.69-7.60) 6.64(3.69-7.60) |
0.6474 |
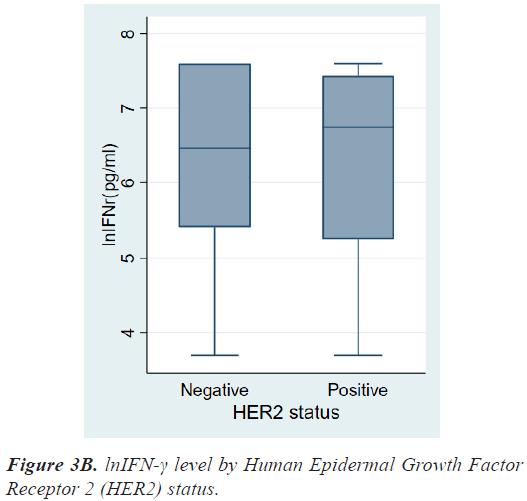
| HER2 status Positive Negative |
6.75(3.69-7.60) 6.46(3.69-7.60) |
0.8976 |
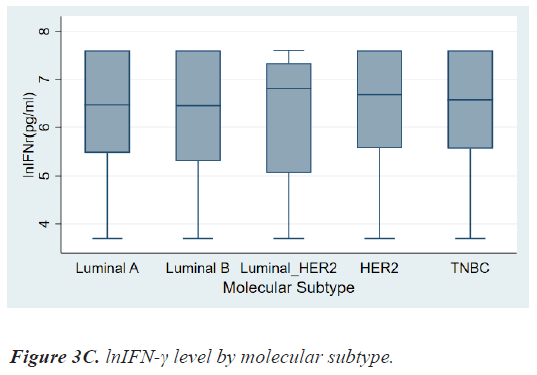
| Subtype Luminal A Luminal B Luminal_HER2 HER2 TNBC |
6.46(3.69-7.60) 6.44(3.69-7.60) 6.80(3.69-7.60) 6.68(3.69-7.60) 6.57(3.69-7.60) |
0.9080 |
Table 2. Analysis of lnIFN- γ according to various patients and tumor related variables.
There were no differences in lnIFN-γ level according to histologic grade and invasion to basement membrane. There were also no differences in lnIFN-γ level according to HR status, HER2 status, and molecular subtypes (Figures 3a-3c).
Discussion
The importance of NK Cells and their association with cancer has been widely documented since early 1980. Through many reports, it is proved that the activity of NK cells controls the antitumor immunity rather than the number of NK cells.
Not all NK cells have the ability to fight abnormal cells, such as cancer cells. Many NK cells lack the ability to eradicate those abnormal cells. Hence, having an increased number of NK cells does not necessarily mean a high antitumor immunity, instead, an increase in NK cell activity reflects a stronger immune system.
Recently, a high-throughput assay was used to measure NKA through detecting NK cell-secreted IFN-γ because IFN-γ secretion represents NK cell activation and effector function [3]. Thus we have evaluated NKA of patients with breast cancer using the same method.
Breast cancer has not traditionally been thought of as highly immunogenic compared to melanoma and nonsmall cell lung carcinoma which have the highest somatic mutational load of all human malignancies [6,7]. However, this study demonstrated that lnIFN-γ level of patients with more progressed breast cancer–LN metastases, distant metastases, advanced stage–was significantly lower than those of relatively less progressed breast cancer. lnIFN-γ levels showed no difference between Ductal Carcinoma In Situ (DCIS) and invasive carcinoma. It could be inferred that NKA is influenced by the regional or systemic spread of tumor cells such as LN metastases or distant metastases rather than local tumor growth. As it is been reported that various immunosuppressive factors produced by macrophages, T lymphocytes, and tumor cells lead to dysfunction of NK cells [8,9].
Among breast cancer subtypes, Triple-Negative Breast Cancer (TNBC) and HER2+ cancer are more immunogenic than HR+/HER2- breast cancer [7,10,11]. In our study, lnIFN-γ level of patients with HR+ breast cancer tended to be lower than that of patients with HR- breast cancer, however, there was no significant statistical difference (6.52, 3.69-7.60 vs. 6.64, 3.69-7.60 pg/ml, P=0.6474).
On the contrary, patients with HER2+ breast cancer had a higher level of lnIFN-γ than those with HER2- breast cancer, although there was also no significant statistical difference (6.75, 3.69-7.60 vs. 6.46, 3.69-7.60 pg/ml, P=0.8976). As a result, lnIFN-γ level of patients with HER2+ cancer was higher than those of Luminal A, however, there was no statistical difference among molecular subtypes.
This study had several limitations. First, it included a relatively small number of patients (N=268), especially the stage 4 patients (n=9). However, the distribution of patients by TNM stage in our study was similar to that of the National Cancer Registry in our country. Second, our study failed to show the statistical differences of lnIFN-γ level by HR status and HER2 status. It seemed to be influenced by sample size, therefore the results might be changed if more patients will be included. However, this study is meaningful because we obtained the results of an inverse association between lnIFN-γ level and TNM stage, especially stage IV.
Conclusion
In conclusion, as the breast cancer stage increases, the level of lnIFN-γ decreases. It is assumed that IFN-γ released from NK cells decreases as cancer progresses. Further studies are needed to analyze the difference of loco-regional recurrence or distant metastasis between the patients with higher IFN-γ level and those with lower IFN-γ level. We suggest that measurement of IFN-γ level released from NK cells can be a useful method of surveillance for breast cancer patients.
Conflicts of Interest
The authors do not have any conflicts of interest to declare.
Acknowledgement
This work was supported by a grant from research year of Inje University in 2017 (subject number 20170092).