ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2018) Volume 29, Issue 4
Retractde: Intracellular NOD2 activation promotes maturation and antigen-presenting functions of dendritic cells exposed to Porphyromonas gingivalis lipopolysaccharide
Han Su1, Xiang Yan2, Wei Chen1, Ting Guo1, Zi-Tong Lin2 and Qin-Gang Hu2*
1Department of Stomatology, Jinling Hospital, Clinical School, Medical College, Nanjing University, Nanjing, PR China
2Nanjing Stomatological Hospital, Medical School of Nanjing University, Nanjing, PR China
- *Corresponding Author:
- Qin-Gang Hu
Nanjing Stomatological Hospital
Medical School of Nanjing University, PR China
Accepted date: November 15, 2017
DOI: 10.4066/biomedicalresearch.29-17-1837
Visit for more related articles at Biomedical ResearchObjective: This study aimed to study the roles of Muramyl Dipeptide (MDP) which is an agonist of NOD2 in Dendritic Cells (DCs) exposed to Porphyromonas gingivalis lipopolysaccharide (P. gingivalis- LPS) on maturation and antigen-presenting functions of DCs to provide experimental evidences to explore the possible mechanism of DCs in periodontitis.
Methods: Flow cytometry was used to detect CD11c, MHC-II, CD80, CD86, and CD40 expression on DCs and ELISA was used to detect IL-12, IFN-γ, IL-10, and IL-13 secreted by DCs which were stimulated by MDP and P. gingivalis-LPS, respectively or in synergism. RT-PCR analysis was used to detect NOD2, TLR2, and TLR4 mRNA expression in DCs stimulated by MDP and P. gingivalis-LPS, respectively or in synergism. CCK8 was used to assess CD4+ T cells proliferation after co-cultured with DCs stimulated by MDP and P. gingivalis-LPS, respectively or in synergism and ELISA was used to detect IL-2, IFN-γ, IL-10 and IL-13 secreted by these T cells.
Results: MDP had weak ability to stimulate DCs maturation but MDP could promote DCs maturation stimulated by P. gingivalis-LPS. MDP was NOD2 agonist to DCs and P. gingivalis- LPS was TLR2 but not TLR4 agonist to DCs. MDP could facilitate TLR2 mRNA expression in DCs exposed to P. gingivalis- LPS. The ability of MDP to promote DCs secreting cytokines was far below P. gingivalis-LPS but MDP could promote the functions of Th2 cell-promoting DCs induced by P. gingivalis-LPS. MDP could promote CD4+T cells proliferation primed by DCs exposed to P. gingivalis-LPS and elevate the ability of DCs exposed to P. gingivalis-LPS to prime Th0 cells to Th2 cells.
Conclusion: Intracellular NOD2 in DCs could be activated by MDP and this activation could promote maturation and the ability to prime Th0 cells to Th2 cells of DCs exposed to P. gingivalis-LPS.
Keywords
Muramyl dipeptide, P. gingivalis-LPS, Dendritic cells, Maturation, Antigen-presenting
Introduction
Dendritic Cells (DCs) are widely distributed in tissues and organs and they are the body's most efficient Antigen- Presenting Cells (APCs) [1]. Immature DCs uptake antigens and mature DCs present antigens to naive T-lymphocytes, then stimulate naive T cells to differentiate to be effector T cells [2], thus, DCs are important key mediators between innate and acquired immune responses [3]. Priming DCs with microbial compounds up-regulates the expression of costimulatory molecules and the production of proinflammatory cytokines, which drives T-helper (Th) cells to differentiate to Th1 or Th2 cells [4]. The first step of the process is that DCs identify various antigenic materials which are called Pathogen- Associated Molecular Patterns (PAMP) by Pattern Recognition Receptors (PRRs) which are expressed either on the surface or in the cytoplasm of DCs. Thus, PRRs and their ligands have important roles in DCs maturation and antigen-presenting function.
Porphyromonas gingivalis (P. gingivalis) is a kind of gramnegative anaerobic rod-shaped bacteria and it is a pathogenic microorganism in the development of Chronic Periodontitis (CP) [5]. The pathogenic components of P. gingivalis include: Lipopolysaccharide (LPS), capsular polysaccharide, fimbrial proteins, and gingipains [6]. P. gingivalis-LPS is one of the main pathogenic factors to periodontitis and P. gingivalis-LPS can elicit various types of immune and inflammatory responses in periodontitis [7]. It is generally accepted that LPS is Toll- Like Receptors (TLR) 4 ligands [8], while there are also many studies declared that different with E. coli-LPS which majorly activated TLR4, P. gingivalis-LPS majorly activated TLR2 [9].
Besides TLRs, there is also another type of important signal transduction PRRs existing in DCs cytoplasm which also play important roles in DCs immune signaling pathway. This kind of PRRs is called nucleotide binding oligomerization domainlike receptors (NLRs) [10]. There are three distinct subfamilies within the NLRs family: the NODs (NOD1-2, NOD3/NLRC3, NOD4/NLRC5, NOD5/NLRX1, CIITA), the NLRPs (NLRP1- 14, also called NALPs), and the IPAF subfamily, consisting of IPAF (NLRC4), and NAIP [11]. Among them, NOD2 are potent to be activated by bacterial cell wall components because NOD2 was revealed to be a receptor for Muramyl Dipeptide (MDP) which is a conserved structure in virtually all types of Peptidoglycan (PGN) which is the main ingredient of bacterial cell wall [12]. That is to say, MDP is the minimal bioactive PGN motif common to all bacteria. Besides, MDP has been shown not to be recognized by TLR2, nor TLR2/1 or TLR2/6 associations [13]. After binding MDP, NOD2 initiate intracellular signalling pathways, which results in the activation of nuclear factor kappa B (NF-κB) and expression of the immune response genes, such as pro-inflammatory cytokines and antimicrobial peptides [14].
Previous study had found that there were DCs in the gingival epithelium tissues of patients with periodontitis [15]. However, the exact mechanisms of these DCs in the development of periodontitis are not clear. Though P. gingivalis has been recognized to be a causal factor in CP and LPS is the main pathogenic component of P. gingivalis, but, as we all know, the oral cavity harbors a diverse and complex microbial community in which many periodontal commensal and pathogenic bacteria live. In the process of bacteria metabolism and periodontal immune reaction, bacteria cell wall ingredient- MDP could be uptaken by immature DCs. These MDP might be recognized as PAMP by NOD2 in the cytoplasm of DCs. However, few studies focused on role of MDP on the functions of DCs.
In this study, we compared the capacities of MDP alone, P. gingivalis-LPS alone and MDP in synergy with P. gingivalis- LPS in the same concentration to induce surface molecules expressed on SD rats’ marrow-derived DCs. We also detected the cytokines released by these DCs and the subsequent Mixed Lymphocyte Reaction (MLR) was examined to confirm whether intracellular NOD2 activation promotes maturation and antigen-presenting functions of DCs exposed to P. gingivalis-LPS.
Materials and Methods
Rats
Six 8 w male SD rats were bred and maintained in a Specific Pathogen Free (SPF) facility in Medical Animal Experimental Center of Nanjing Command, PLA. All protocols of animal studies were approved by Nanjing University Committee on Use and Care of Animals.
Reagents
Roswell Park Memorial Institute (RPMI) 1640 medium, heatinactivated fetal bovine serum, streptomycin and penicillin(all from HyClone), recombinant rat granulocyte macrophage colony-stimulating factor (rrGM-CSF), recombinant rat interleukin-4 (rrIL-4) (both from R&D), standard P. gingivalis- LPS (Originating from Porphyromonas gingivalis, cat.: tlrlpglps), MDP (both purchased from Invivogen), Phycoerythrin (PE)-conjugated anti-CD11c, Allophycocyanin (APC)- conjugated anti-MHCII, Fluorescein Isothiocyanate (FITC)- conjugated anti-CD86, APC-conjugated anti-CD80 and FITCconjugated anti-CD40 anti-rat monoclonal antibody (all from Biolegend), Interferon-γ (IFN-γ), IL-12, IL-2, IL-10, and IL-13 rat ELISA kits (R&D Systems), CD4 anti-rat monoclonal antibody beads (Miltenyi Biotec), Cell Counting Kit-8 (Dojindo).
Apparatus
FACSCalibur Cytometer (Becton Dickinson, BD Biosciences), SpectraMax M3 Automatic enzyme-labelling measuring instrument (Fortune Lab systems), MidiMACS Starting Kit (Miltenyi Biotec).
Cell culture
Total bone marrow cells were freshly isolated from tibias, femurs and humerus of the rats. Cells were cultured in complete RPMI 1640 medium supplemented with 10% heatinactivated Fetal Bovine Serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin, supplemented with 10 ng/ml rrGMCSF and 1 ng/ml rrIL-4 to generate DCs. Cells were incubated at 37°C in a humidified 5% CO2 incubator. Culture media were half changed every other day in which all the reagents concentrations were kept and suspension cells were collected. After 6 d culture, the harvested cells were 2 × 106 per well in 6-well plates and randomly divided into seven groups: control group, 50 ng/ml MDP group, 100 ng/ml MDP group, 50 ng/ml P. gingivalis-LPS group, 100 ng/ml P. gingivalis-LPS group, 50 ng/ml MDP+50 ng/ml P. gingivalis-LPS group and 100 ng/ml MDP+100 ng/ml P. gingivalis-LPS group. After 48 h culture, loosely adhered cells and cells suspension were collected. CD11c expressed on cell surface was detected by flow cytometry. The results showed that CD11c+ cells population was >90 %, then these cells were harvested as DCs.
Cells surface molecules expressions analysis
On the eighth cell culture day, the cells of each group were collected, counted and washed by PBS, then resuspended to 5.0 × 105/ml and transferred to flow tubes. According to the groups, PE-CD11c, APC-MHCII, FITC-CD86, APC-CD80 and FITC-CD40 anti-rat monoclonal antibodies were added to the flow tubes according to the manufacturer instructions to detect double-labeled of CD11c+MHCII+, CD11c+CD80+, CD11c+CD86+, CD11c+CD40+ cells by flow cytometry. Isotype-matched control antibodies were added to the negative control tubes of each group. Cells were incubated at 4°C in the dark for 30 min, then washed twice by PBS and fully resuspended. The expressions of surface molecules on DCs were detected by FACS Calibur. The raw data obtained in experiments were processed by FlowJo7.6.1 software to analyse the percentage of positive cells in each group. Assays were performed in triplicate for each independent experiment.
Real-time polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from DCs cultured by TriReagent (Sigma) as prescribed by the manufacturer. Purified RNA was reverse-transcribed with Superscript II RNase H (Invitrogen) according to the manufacturer's protocol. The generated cDNA was amplified by using primers for rat NOD2 (forward-primer: 5'-GGCATCAAACTGTACCTGAGAAAGC-3', reverseprimer: 5'-TTGGGAAGCCCCTGTTCTGTAG-3'), rat TLR2 (forward-primer: 5'-GGAGAACTCTGACCCACCCTT-3', reverse-primer: 5'-AAGAGCCTGAAGTGGGAGAAGT-3'), rat TLR4 (forward-primer: 5'- TGCTCTGGGGAGGCACATCT-3', reverse-primer: 5'- CCCTGTGAGGTCGTTGAG GTTAG-3') and glyceraldehyde-3-phosphatedehydrogenase (GAPDH, forwardprimer: 5'-AGTGCCAGCCTCGTCTCATAG-3', reverseprimer: 5'-CGTTGAACTTGCCGTGGGTAG-3'). The cDNA samples were heat denatured (95°C for 10 min) and then amplified with 35 cycles, each comprising successive incubations at 95°C for 15 s, 60°C for 35 s and 72°C for 35 s. A further extension step was done at 72°C for 5 min. The amplicons (GAPDH: 192 bp, NOD2: 221 bp, TLR2: 186 bp, TLR4: 244 bp) were size fractionated onto a 2% agarose gel and stained with ethidium bromide. Relative Expression (RE) of each gene of interest was calculated by the formula:
RE=2-ΔΔCt
Cytokines detection
On the eighth cell culture day, to measure cytokines secreted by DCs, the supernatants of each group were collected. The levels of the cytokines: IFN-γ, IL-12, IL-10, and IL-13 in the culture supernatants were measured by ELISA according to the manufacturer’s protocol and the absorbance was read at 450 nm. Assays were performed in triplicate for each independent experiment.
Mixed lymphocyte reaction (MLR) and allogeneic Tcell proliferation and activation assay
To determine the capacity of DCs to prime allogeneic CD4+T cells, SD rats’ splenocytes were obtained and CD4+T cells were further purified by using a CD4+T cell magnetic bead sorting kit according to the manufacturer’s instructions. In all of the experiments, the isolated cells were 90-95% CD4+ as determined by staining with PE-conjugated anti-CD4 antibody followed by flow cytometry (data not shown). Dendritic cells of each group as described above were cocultured with 2 × 105 CD4+T cells at the ratio of 1:10, in triplicates, in 96-well plate to ensure efficient DC/T cells
Contact: RPMI-1640 medium supplemented with 10% heatinactivated FBS and 100 U/ml penicillin and 100 μg/ml streptomycin in a total volume of 200 μl was added to each well. Cells were placed in 37°C, humidified incubator with 5% CO2 atmosphere for 72 h. 4 h before the end, 10 μl Cell Counting Kit-8 (CCK-8) was added to each well according to the manufacturer’s instructions and incubated for 4 h, then the absorbance was read at 450 nm to measure allogeneic T-cells proliferation. Assay was repeated three times.
As the method described above, DCs of each group were cocultured with CD4+T cells at the ratio of 1:10 for 72 h. Cytokines of IFN-γ, IL-2, IL-10 and IL-13 in the supernatant were measured in triplicate by ELISA, following the manufacturer’s instructions. The absorbance was read at 450 nm.
Statistical analysis
Data were expressed as mean ± SD (Standard Deviation).Twosided Student t test was used to determine statistically significant differences between two groups. One-way Analysis of Variance (ANOVA) followed by Dunnett’s multiple comparison tests was used to analyse differences among multiple groups. Differences between groups were considered statistically significant when the p value was<0.05.
Results
MDP could promote DCs maturation simulated by P. gingivalis-LPS
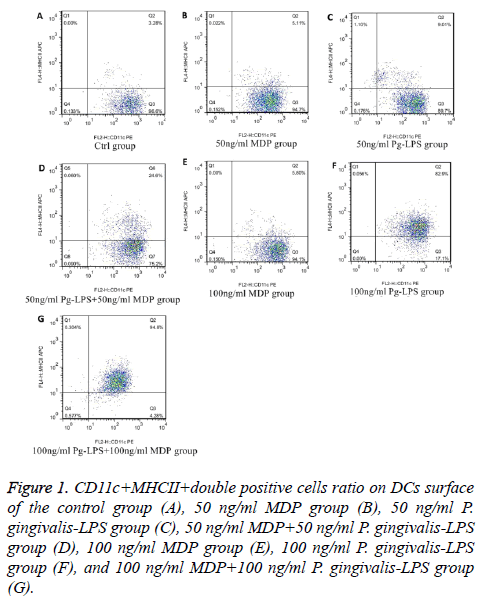
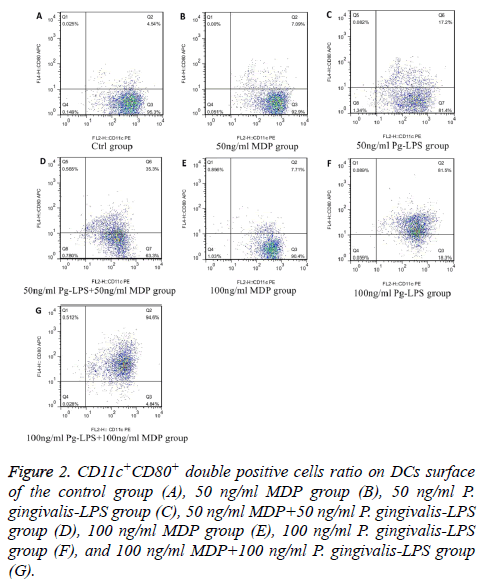
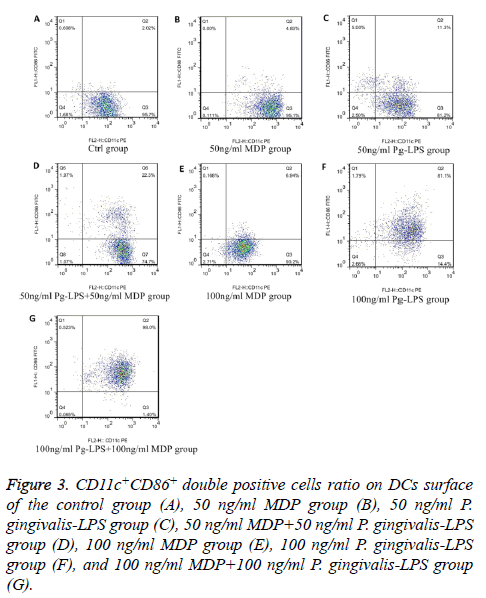
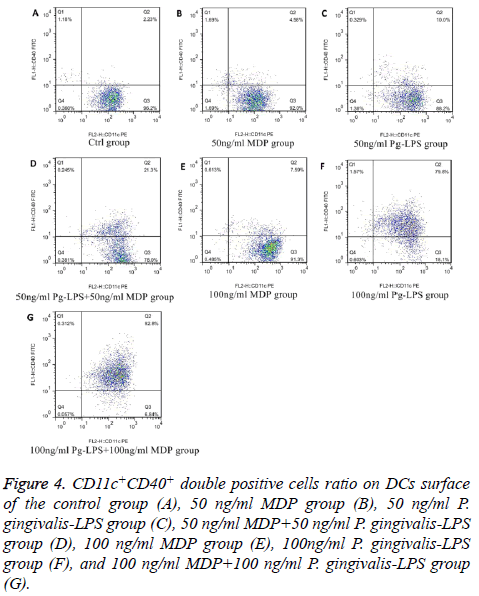
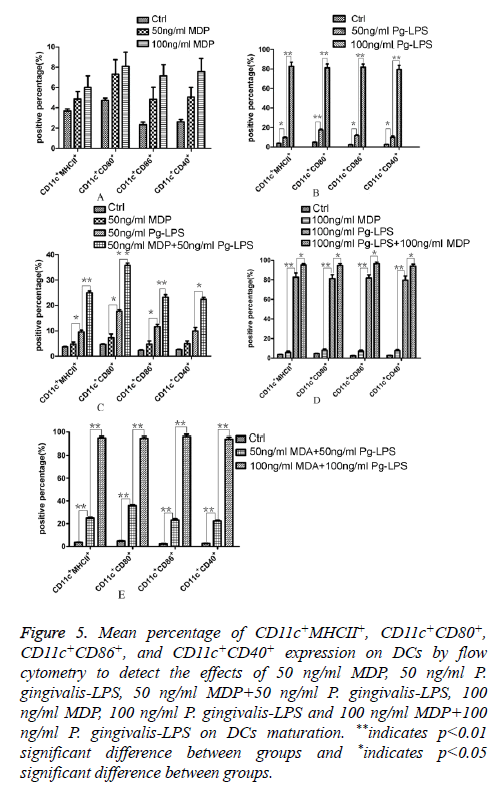
We analysed the levels of surface molecules expression to detect the effects of DCs maturation in groups of control, 50 ng/ml MDP, 100 ng/ml MDP, 50 ng/ml P. gingivalis-LPS, 100 ng/ml P. gingivalis-LPS, MDP+50 ng/ml P. gingivalis-LPS and 100 ng/ml MDP+100 ng/ml P. gingivalis-LPS by means of flow cytometry. CD11c+MHCII+, CD11c+CD80+, CD11c +CD86+ and CD11c+CD40+ cells in each group were shown in Figures 1-4. As the results shown in the histogram of Figure 5, compared with the control group, neither 50 ng/ml MDP nor 100 ng/ml MDP could stimulate DCs to undergo maturation (P>0.05). The elevation of MDP concentration from 50 ng/ml to 100 ng/ml could not significantly promote DCs maturation (P>0.05) (Figure 5A). Unlike MDP, 50 ng/ml P. gingivalis-LPS could elevate MHCII, CD80, CD86 and CD40 expressed on DCs surface and the elevation of P. gingivalis-LPS concentration from 50 ng/ml to 100 ng/ml could significantly promote DCs maturation (P<0.01) (Figure 5B). Figures 5C and 5D indicated that the ability of MDP to stimulate DCs maturation was weaker than that of P. gingivalis-LPS in the same concentration (P<0.05). In both concentrations of 50 ng/ml and 100 ng/ml, being compared with individual function of P. gingivalis-LPS, synergism with MDP could apparently up-regulated all the levels of MHCII, CD80, CD86, CD40 expressed on DCs (P<0.05). All the above results showed that MDP had weak ability to stimulate DCs maturation but MDP could promote DCs maturation stimulated by P. gingivalis- LPS.
Figure 1: CD11c+MHCII+double positive cells ratio on DCs surface of the control group (A), 50 ng/ml MDP group (B), 50 ng/ml P. gingivalis-LPS group (C), 50 ng/ml MDP+50 ng/ml P. gingivalis-LPS group (D), 100 ng/ml MDP group (E), 100 ng/ml P. gingivalis-LPS group (F), and 100 ng/ml MDP+100 ng/ml P. gingivalis-LPS group (G).
Figure 2: CD11c+CD80+ double positive cells ratio on DCs surface of the control group (A), 50 ng/ml MDP group (B), 50 ng/ml P. gingivalis-LPS group (C), 50 ng/ml MDP+50 ng/ml P. gingivalis-LPS group (D), 100 ng/ml MDP group (E), 100 ng/ml P. gingivalis-LPS group (F), and 100 ng/ml MDP+100 ng/ml P. gingivalis-LPS group (G).
Figure 3: CD11c+CD86+ double positive cells ratio on DCs surface of the control group (A), 50 ng/ml MDP group (B), 50 ng/ml P. gingivalis-LPS group (C), 50 ng/ml MDP+50 ng/ml P. gingivalis-LPS group (D), 100 ng/ml MDP group (E), 100 ng/ml P. gingivalis-LPS group (F), and 100 ng/ml MDP+100 ng/ml P. gingivalis-LPS group (G).
Figure 4: CD11c+CD40+ double positive cells ratio on DCs surface of the control group (A), 50 ng/ml MDP group (B), 50 ng/ml P. gingivalis-LPS group (C), 50 ng/ml MDP+50 ng/ml P. gingivalis-LPS group (D), 100 ng/ml MDP group (E), 100ng/ml P. gingivalis-LPS group (F), and 100 ng/ml MDP+100 ng/ml P. gingivalis-LPS group (G).
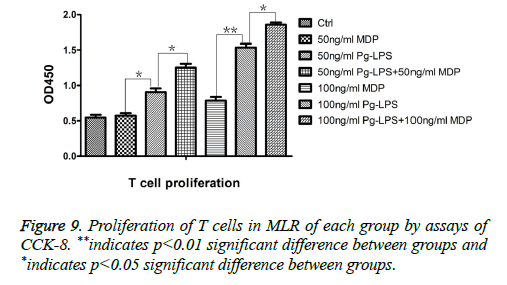
Figure 5: Mean percentage of CD11c+MHCII+, CD11c+CD80+, CD11c+CD86+, and CD11c+CD40+ expression on DCs by flow cytometry to detect the effects of 50 ng/ml MDP, 50 ng/ml P. gingivalis-LPS, 50 ng/ml MDP+50 ng/ml P. gingivalis-LPS, 100 ng/ml MDP, 100 ng/ml P. gingivalis-LPS and 100 ng/ml MDP+100 ng/ml P. gingivalis-LPS on DCs maturation. **indicates p<0.01 significant difference between groups and *indicates p<0.05 significant difference between groups.
MDP could promote TLR2 mRNA expression in DCs exposed to P. gingivalis-LPS
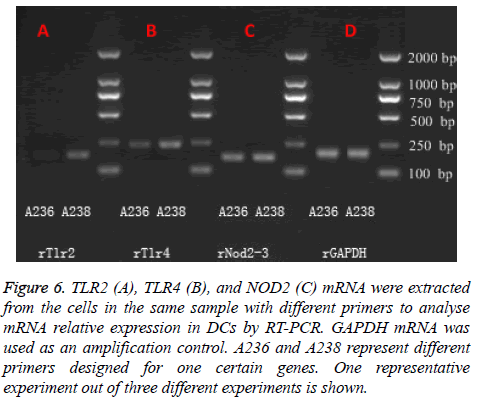
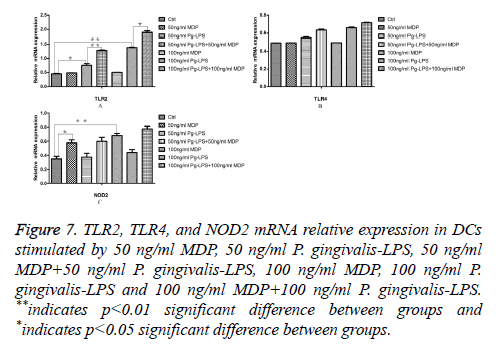
There are many contradictory viewpoints of whether TLR2 or TLR4 is functional and plays key roles in different cell lines exposed to P. gingivalis-LPS. So in the present study, we examined the expression of TLR2, TLR4, and NOD2 mRNA in DCs in each group by RT-PCR. Figure 6 shows the result of TLR2, TLR4, and NOD2 mRNA analysis of DCs from the same sample with different primers. All cells express mRNA coding for TLR2, TLR4, and NOD2. Figure 7 showed the results of TLR2, TLR4 and NOD2 mRNA analysis in DCs. Being compared with the control group, 50 ng/ml P. gingivalis- LPS could promote TLR2 mRNA expression in DCs (P<0.05) and 100 ng/ml P. gingivalis-LPS could highly promote TLR2 mRNA expression in DCs (P<0.01). Besides, being compared with P. gingivalis-LPS acted solely, both 50 ng/ml MDP in synergy with 50 ng/ml P. gingivalis-LPS and 100 ng/ml MDP in synergy with 100 ng/ml P. gingivalis-LPS could facilitate TLR2 mRNA expression in DCs (P<0.05, Figure 7A). A weak TLR4 response to P. gingivalis-LPS could be found in DCs due to low expression of TLR4 (Figure 7B). Being compared with the control group, 50 ng/ml MDP could promote NOD2 mRNA expression in DC (P<0.05) and 100 ng/ml MDP could highly promote NOD2 mRNA expression in DCs (P<0.01, Figure 7C). Together, these data suggested that MDP was NOD2 agonist to DCs and P. gingivalis-LPS was TLR2 but not TLR4 agonist to DCs. MDP could facilitate TLR2 mRNA expression in DCs exposed to P. gingivalis-LPS.
Figure 6: TLR2 (A), TLR4 (B), and NOD2 (C) mRNA were extracted from the cells in the same sample with different primers to analyse mRNA relative expression in DCs by RT-PCR. GAPDH mRNA was used as an amplification control. A236 and A238 represent different primers designed for one certain genes. One representative experiment out of three different experiments is shown.
Figure 7: TLR2, TLR4, and NOD2 mRNA relative expression in DCs stimulated by 50 ng/ml MDP, 50 ng/ml P. gingivalis-LPS, 50 ng/ml MDP+50 ng/ml P. gingivalis-LPS, 100 ng/ml MDP, 100 ng/ml P. gingivalis-LPS and 100 ng/ml MDP+100 ng/ml P. gingivalis-LPS. **indicates p<0.01 significant difference between groups and *indicates p<0.05 significant difference between groups.
MDP could promote the secretion of IL-10 and IL-13 by DCs exposed to P. gingivalis-LPS
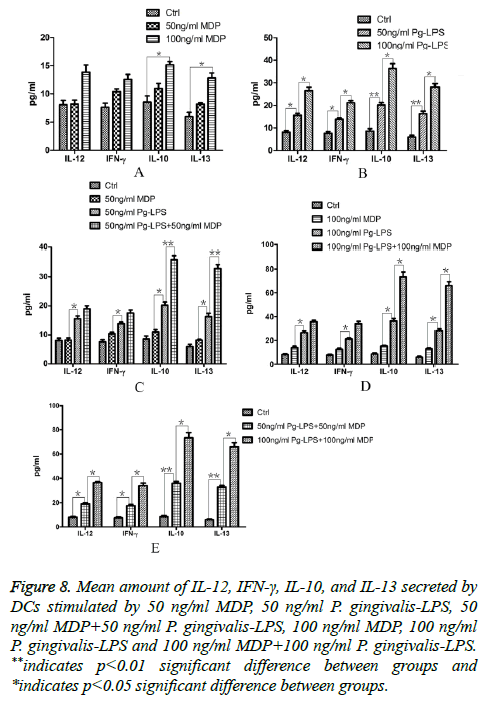
As the cytokines produced by DCs affect subsequent DCs function, we investigated MDP function on the aspect of inducing DCs exposed to P. gingivalis-LPS to secrete cytokines. We analysed the culture supernatants from DCs of each group and the productions of IFN-γ, IL-12, IL-10, and IL-13 were examined by ELISA. The results were shown as Figure 8. It was indicated that compared with the control group, 50 ng/ml MDP couldn’t promote all the four cytokines secreted by DCs (P>0.05) and 100 ng/ml MDP could only promote IL-10 and IL-13 secreted by DCs (P<0.05). The elevation of MDP concentration from 50 ng/ml to 100 ng/ml couldn’t increase all the four cytokines secreted by DCs (p>0.05, Figure 8A). Meanwhile, 50 ng/ml P. gingivalis-LPS could promote all the four cytokines secreted by DCs (p<0.05) and the elevation of P. gingivalis-LPS concentration from 50 ng/ml to 100 ng/ml could promote all the four cytokines secreted (p<0.05, Figure 8B). All the four cytokines secreted by DCs in MDP group were far lower than those in P. gingivalis-LPS with the same concentration group (P<0.05). In both concentrations of 50 ng/ml and 100 ng/ml, MDP could promote IL-10 and IL-13 secreted by DCs exposed to P. gingivalis-LPS (P<0.05) but MDP couldn’t promote IL-12 and IFN-γ secreted by DCs exposed to P. gingivalis-LPS (P>0.05, Figures 8C and 8D). All these findings demonstrated that the ability of MDP to promote DCs secreting cytokines was far below that P. gingivalis-LPS but MDP could promote the functions of Th2 cell-promoting DCs induced by P. gingivalis- LPS.
Figure 8: Mean amount of IL-12, IFN-γ, IL-10, and IL-13 secreted by DCs stimulated by 50 ng/ml MDP, 50 ng/ml P. gingivalis-LPS, 50 ng/ml MDP+50 ng/ml P. gingivalis-LPS, 100 ng/ml MDP, 100 ng/ml P. gingivalis-LPS and 100 ng/ml MDP+100 ng/ml P. gingivalis-LPS. **indicates p<0.01 significant difference between groups and *indicates p<0.05 significant difference between groups.
MDP could promote CD4+T cells proliferation primed by DCs exposed to P. gingivalis-LPS and elevate the ability of DCs exposed to P. gingivalis-LPS to prime Th0 cells to Th2 cells.
The results of CCK8 indicated that in the aspect to prime CD4+T cells proliferation, there was no significant difference between DCs in control group, DCs in 50 ng/ml MDP group and DCs in 100 ng/ml MDP group (P>0.05). That is to say, promoting the concentration of MDP from 50 ng/ml to 100 ng/ml couldn’t promote the ability of MDP to prime DCs. In both concentrations of 50 ng/ml and 100 ng/ml, the ability of MDP to prime DCs was weaker than P. gingivalis-LPS in the same concentration (P<0.05). But in both concentrations of 50 ng/ml and 100 ng/ml, MDP could significantly promote the ability of DCs exposed to P. gingivalis-LPS to prime CD4+T cell proliferation (P<0.05). That is to say, MDP had the synergistic effect with P. gingivalis-LPS in the aspect of promoting antigen-presenting functions of DCs (Figure 9).
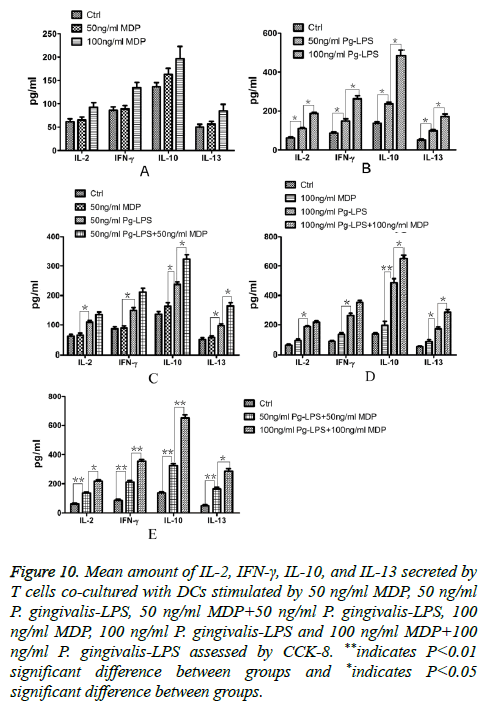
As different effector T cells could secrete different predominant cytokines, we investigated IL-2, IFN-γ, IL-10, and IL-13 secreted by activated CD4+T cells co-cultured with DCs in the culture supernatants of each group. The results were shown as Figure 10. Compared with the control group, neither 50 ng/ml MDP nor 100 ng/ml MDP could facilitate all the four cytokines secretion (P>0.05, Figure 10A). Meanwhile, 50 ng/ml P. gingivalis-LPS could elevate all the four cytokines secretion and the elevation of P. gingivalis-LPS concentration from 50 ng/ml to 100 ng/ml also facilitated all the four cytokines secretion (P<0.05, Figure 10B). All the four cytokines secreted by T cells in MDP group were far below those in P. gingivalis-LPS in the same concentration group (P<0.05). In both concentrations of 50 ng/ml and 100 ng/ml, MDP could promote IL-10 and IL-13 secreted by T cells primed by DCs exposed to P. gingivalis-LPS (P<0.05) but MDP couldn’t promote IL-2 and IFN-γ secreted by T cells primed by DCs exposed to P. gingivalis-LPS (P>0.05, Figures 10C and 10D). These results were in accordance with the differential cytokines secreted by DCs in response to MDP, P. gingivalis-LPS and MDP synergized with P. gingivalis-LPS. They revealed that MDP could elevate the ability of DCs exposed to P. gingivalis-LPS to prime Th0 cells to Th2 cells.
Figure 10: Mean amount of IL-2, IFN-γ, IL-10, and IL-13 secreted by T cells co-cultured with DCs stimulated by 50 ng/ml MDP, 50 ng/ml P. gingivalis-LPS, 50 ng/ml MDP+50 ng/ml P. gingivalis-LPS, 100 ng/ml MDP, 100 ng/ml P. gingivalis-LPS and 100 ng/ml MDP+100 ng/ml P. gingivalis-LPS assessed by CCK-8. **indicates P<0.01 significant difference between groups and *indicates P<0.05 significant difference between groups.
Discussion
PRRs could recognize conserved structures present on microorganisms as PAMPs. These receptors are mainly expressed by immune cells [16]. The two best-characterized classes of PRRs are TLRs and NLRs. TLRs sense the presence of microbial molecules on the cell surface and in endosomes, and NLRs recognize microbial molecules in the cytosol [17]. One of the major functions of the PRRs is to signal the APCs to induce the release of particular chemokines and cytokines, which in turn determine the nature of the acquired immune responses [18]. Inside NLRs family, NOD2 recognizes the PGN derivative-MDP and then induces immune responses [19]. Inside TLRs family, TLR4 is generally accepted as LPS receptor. But there are also many contradictory conclusions declared that different from E. coli-LPS which was generally used in researches, main periodontal pathogenic component, P. gingivalis-LPS had differential roles with E. coli-LPS in different cell lines [20].
DCs are professional APCs involved in the initiation and polarization of the acquired immune responses. The priming of Th cell subsets could be orchestrated by cytokines produced by DCs that sense PAMPs [21]. Previous study had found that there were DCs in the gingival epithelium tissues of patients with periodontitis [15]. Besides, studies also revealed that CD4+T cells infiltrated to gingival tissue and expressed the mRNA of IL-10, IFN-γ, IL-6, and IL-13 which were associated with periodontal diseases [5]. These results suggested that DCs and T cells may be actively involved in the immunopathogenesis of periodontitis. What are the interactions of DCs and T cells during periodontitis? The results of this research revealed the effects of P. gingivalis-LPS and MDP in the maturation of DCs and revealed the ability of DCs to present these periodontal microorganisms antigens to CD4+ T cells.
The results of PCR revealed that P. gingivalis-LPS could elevate the expression of TLR2 mRNA in DCs, but could not elevate the expression of TLR4 mRNA in DCs. These results meant that P. gingivalis-LPS was the agonist of TLR2 in DCs, which were in accordance with our previous discovery that different from E. coli-LPS that is the agonist of TLR4, P. gingivalis-LPS is the agonist of TLR2 in DCs [22]. All these differences between P. gingivalis-LPS and E. coli-LPS might rely on that there were unique and heterogenous chemical structures in hydrophilic bisphosphate frames of lipid A between P. gingivalis-LPS and E. coli-LPS and these structural differences might lead to differences between P. gingivalis-LPS and E. coli-LPS in affinity to TLRs [23].
In the present experiment, we used MDP and P. gingivalis-LPS to act on bone marrow-derived DCs, and the results showed that the ability of MDP to promote DCs maturation and antigen presenting function was weaker than P. gingivalis-LPS in the same concentration. Compared with P. gingivalis-LPS used solo, MDP in synergy with P. gingivalis-LPS could obviously promote the maturation of DCs. MDP in synergy with P. gingivalis-LPS can promote IL-10 and IL-13 but not IL-12 and IFN-γ secreted by DCs, which indicated that MDP could promote DCs exposed to P. gingivalis-LPS to secrete Th2 cytokines. The MLR results of DCs co-cultured with CD4+T cells showed that MDP in synergy with P. gingivalis-LPS promoted the proliferation of T cells. Besides, MDP in synergy with P. gingivalis-LPS could facilitate T cells to secrete IL-10 and IL-13 but not IL-2 and IFN-γ. That was to say, DCs stimulated by MDP in synergy with P. gingivalis-LPS could promote the differentiation of Th0 cells to Th2 cells. Obviously, MLR results were consistent with the aforementioned results that MDP in synergy with P. gingivalis- LPS could promote DCs to secrete Th2 cytokines.
The downstream adaptor protein of NOD1/2 is RIP2 and the downstream adaptor protein of TLR2/TLR4 is MyD88. The downstream pathway of both MyD88 and RIP2 is the NF-κB pathway, so the NOD1/2 ligands could be synergistic with TLR2/TLR4 ligands [24]. Presently, in the aspect of the synergistic effect of NOD1/2 and TLR ligands, the coordination of NOD1/2 and TLR4 ligands was mostly researched. Selvanantham researches revealed a synergy between Nod1/2 and TLR4 in DCs that potentiates IL-12 production [17]. Tada declared that MDP synergy with TLR4 ligand-lipid A could promote IL-12 and IFN-γ secreted by human DCs to induce Th1 reaction [25]. Fritz found that MDP synergy with E. coli-LPS could promote human DCs maturation and promote IL-6, IL-8, IL-12 and TNF-α secreted by DCs [26]. In the aspect of the synergistic effect of TLR2 ligand and NOD2 ligand, Watanabe T pointed out that the TLR2 ligand-PGN cooperated with MDP to inhibit IL-10 and IL-12 secreted by Card15-/- mouse spleen cells, and ultimately inhibit Th1 reaction mediated by TLR2 [27]. Hiemstra research found that intestinal epithelial cells prior exposure to MDP significantly increased the production of chemokines and cytokines and improved the barrier function induced by different TLR2 and TLR4 ligands [28]. In the present study, we found that NOD2 ligand-MDP could improve maturation and antigen-presenting functions of DCs exposed to TLR2 ligand- P. gingivalis-LPS. The mechanism of the function might rely on that MDP prime synergistic induction of inflammatory signaling cascade with TLR2 activated by P. gingivalis-LPS and this synergistic effect might promote maturation and antigen-presenting functions of DCs. However, the study also has some limitations, a deeper insight of NOD2 ligand-MDP on DCs, for example if there are other pathways involved in the process, is still needed to be further revealed.
Present study explored DCs immune responses induced by P. gingivalis-LPS and MDP. The study suggested that in the periodontitis environment that many microorganism exits, one of the potent mechanisms of periodontitis might be the alteration of DCs functions stimulated by P. gingivalis-LPS which is the main pathogenic factor of periodontal diseases, accompanied by many other microorganism components. That might also be the key factor in periodontal cellular immunity and the participant factor in periodontal humoral immunity. Then, periodontal specific immunity together with periodontal nonspecific immunity leads to periodonitis. Besides, our study revealed the differential roles of P. gingivalis LPS and E. coli LPS in maturation and antigen-presenting functions of dendritic cells. These results may help to further elucidate the possible mechanism of DCs in the occurrence and development of periodontitis and provide new approach to periodontal immunotherapy.
References
- Hancock DG, Guy TV, Shklovskaya E, Fazekasd SGB. Experimental models to investigate the function of dendritic cell subsets: challenges and implications. Clin Exp Immunol 2013; 171: 147.
- Johnson KE, Redd AD, Quinn TC, Collinsonstreng AN, Cornish T, Kong X. Effects of HIV-1 and herpes simplex virus type 2 infection on lymphocyte and dendritic cell density in adult foreskins from Rakai, Uganda. Journal of Infect Dis 2011; 203: 602-609.
- Hammad H, Lambrecht BN. Dendritic cells and airway epithelial cells at the interface between innate and adaptive immune responses. Allergy 2011; 66: 579-587.
- Bajwa A, Huang L, Ye H, Dondeti K, Song S, Rosin DL. Dendritic cell sphingosine 1-phosphate receptor-3 regulates th1-th2 polarity in kidney ischemia-reperfusion injury. J Immunol 2012; 189: 2584.
- Moutsopoulos NM, Kling HM, Angelov N, Jin W, Palmer RJ, Nares S. Porphyromonas gingivalis promotes Th17 inducing pathways in chronic periodontitis. J Autoimmun 2012; 39: 294-303.
- Holt SC, Kesavalu L, Walker S, Genco CA. Virulence factors of Porphyromonas gingivalis. Periodontology 2000 1999; 20: 168-238.
- Jain S, Darveau RP. Contribution of Porphyromonas gingivalis lipopolysaccharide to periodontitis. Periodontology 2010; 54: 53-70.
- Stevens CW, Aravind S, Das S, Davis RL. Pharmacological characterization of LPS and opioid interactions at the toll-like receptor 4. Br J Pharmacol 2013; 168: 1421.
- Sun Y, Li H, Sun MJ, Zheng YY, Gong DJ, Xu Y. Endotoxin tolerance induced by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli: alternations in Toll-like receptor 2 and 4 signaling pathway. Inflamm 2014; 37: 268.
- Nemazee D, Gavin A, Hoebe K, Beutler B. Toll-like receptors and antibody responses. Nature 2006; 441: 4.
- Berrington WR, Smith KD, Skerrett SJ, Hawn TR. Nucleotide-binding oligomerization domain containing-like receptor family, caspase recruitment domain (CARD) containing 4 (NLRC4) regulates intrapulmonary replication of aerosolized Legionella pneumophila. BMC Infect Dis 2013; 13: 371.
- Duerr CU, Salzman NH, Dupont A, Szabo A, Normark BH, Normark S. Control of intestinal Nod2-mediated peptidoglycan recognition by epithelium-associated lymphocytes. Mucosal Immunol 2011; 4: 325-334.
- Uehori J, Fukase K, Akazawa T, Uematsu S, Akira S, Funami K. Dendritic cell maturation induced by muramyl dipeptide (MDP) derivatives: monoacylated MDP confers TLR2/TLR4 activation. J Immunol 2005; 174: 7096-7103.
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature 2012; 481: 278-286.
- Bodineau A, Coulomb B, Tedesco AC, Seguier S. Increase of gingival matured dendritic cells number in elderly patients with chronic periodontitis. Arch Oral Biol 2009; 54: 12-16.
- Krishnaswamy JK, Chu T, Eisenbarth SC. Beyond pattern recognition: NOD-like receptors in dendritic cells. Trends Immunol 2013; 34: 224-233.
- Selvanantham T, Escalante NK, Cruz TM, Fieve S, Girardin SE, Philpott DJ. Nod1 and Nod2 enhance TLR-mediated invariant NKT cell activation during bacterial infection. J Immunol 2013; 191: 5646-5654.
- Diebold SS. Activation of dendritic cells by toll-like receptors and C-type lectins. Handbook Exp Pharmacol 2009; 3-30.
- Majewska-Szczepanik M, Dorozynska I, Strzępa A, Szczepanik M. Epicutaneous immunization with protein antigen TNP-Ig and NOD2 ligand muramyl dipeptide (MDP) reverses skin-induced suppression of contact hypersensitivity. Pharmacol Rep 2014; 66: 137-142.
- Jones KJ, Ekhlassi S, Montufar-Solis D, Klein JR, Schaefer JS. Differential cytokine patterns in mouse macrophages and gingival fibroblasts after stimulation with porphyromonas gingivalis or Escherichia coli lipopolysaccharide. J Periodontol 2010; 81: 1850.
- Souto GR, Queirozjunior CM, Costa FO, Mesquita RA. Pro-inflammatory, Th1, Th2, Th17 cytokines and dendritic cells: a cross-sectional study in chronic periodontitis. Plos One 2014; 9: 91636.
- Su H, Yan X, Dong Z, Chen W, Lin ZT, Hu QG. Differential roles of Porphyromonas gingivalis lipopolysaccharide and Escherichia coli lipopolysaccharide in maturation and antigen-presenting functions of dentritic cells. Eur Rev Med Pharmacol Sci 2015; 19: 2482-2492.
- Andrukhov O, Ertlschweiger S, Moritz A, Bantleon HP, Rauschfan X. Different effects of P. gingivalis LPS and E. coli LPS on the expression of interleukin-6 in human gingival fibroblasts. Acta Odontologica Scandinavica 2014; 72: 337-345.
- Salem M, Seidelin JB, Eickhardt S, Alhede M, Rogler G, Nielsen OH. Species-specific engagement of human NOD2 and TLR signaling upon intracellular bacterial infection: Role of Crohns associated NOD2 gene variants. Clin Exp Immunol 2015; 179: 426-434.
- Tada H, Aiba S, Shibata KI, Ohteki T, Takada H. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and t helper type 1 cells. Infect Immun 2005; 73: 7967.
- Fritz JH, Girardin SE, Fitting C, Werts C, Mengin LD, Caroff M. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur J Immunol 2005; 35: 2459.
- Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol 2004; 5: 800-808.
- Hiemstra IH, Bouma G, Geerts D, Kraal G, Haan JMMD. Nod2 improves barrier function of intestinal epithelial cells via enhancement of TLR responses. Mol Immunol 2012; 52: 264-272.