ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 15
Iontophoretic delivery of transdermal patches containing lignocaine with clonidine and epinephrine in healthy volunteers: Effect of concentration of epinephrine and clonidine on local anesthesia
Li Xiao and Tianzuo Li*
Department of Anesthesiology, Beijing Shijitan Affiliated Hospital of the Capital Medical University, Beijing 100038, PR China
- *Corresponding Author:
- Tianzuo Li
Department of Anesthesiology
Beijing Shijitan Affiliated Hospital of the Capital Medicald
University, PR China
Accepted on June 15, 2017
The ultimate aim of the initiating the study was find out effect of admixture of Clonidine and/or Epinephrine on Lignocaine. After co- administering clonidine (150 μg, n=20) or Epinephrine (200 μg, n=20) to Lignocaine, end points like sensory or motor blockade, depth of anesthesia, cool and hot sensation, plasma level of Lignocaine was studied. Total 60 subjects received the combination aforesaid formulation via iontophoretic patch. The prepared patches matched with standard measures required for transdermal delivery. The subjects in different groups shows resemblance in terms of age, height, body weight etc. Sedation score of motor or sensory blockade showed indifferent results. Only few subjects (n=2) showed complete freedom from any type of pain (3.34%) on administration of Epinephrine. VAS score in range of 0-7 was observed with that of clonidine (range 1-8). In subjects with clonidien plasma level concentration of Lignocaine found to be higher (3.2 μmol/L.) when compared to epinephrine (2.6 μmol/L) due to lack of vasoconstriction effect in epinephrine. This increase availability of Lignocaine reduces margin of safety with clonidine. Mild type of irritation persisting only for 2-5 min was observed in few patients entering the trial. No patient involved report swelling, itching, edema.
Keywords
Lignocaine, Epinephrine, Clonidine, Iontophoresis, transdermal patch.
Introduction
The presence of stratum corneum as the outermost layer produces great challenge for investigators thus making topical delivery a little tedious task [1]. Despite the fact the system is uttermost favorite due to the significant clinical benefits including controlled release rate, no pain, maintaining steady state plasma level and uppermost is avoidance of hepatic first pass metabolism [2]. The aforesaid effects can be advanced by making use of various penetration enhancers (thereby to make upper stratum corneum permeable) for lipophilic drug exhibiting high potency despite of low molecular weight. Iontophoresis (IP) is recently accepted and implemented physical technique showing its effects on direct stratum corneum [3]. The technique deals with the transferring charged molecules into/ through biological membranes or tissues with slight electric current. These current aids in movement of ion across biological membrane using externally applied potential difference [4]. The application of very low voltage (approx. 0.1-5 V) and constant current (0.5 mA/cm2) for specifies period of time induces transportation of drug molecule and/or salt of drug molecule through skin. The availability or distribution of drug after treatment depends upon the factors like charge of ion, current intensity, surface of electrode exposed, concentration of drug etc [4,5]. When the membrane involved in this is skin then the process usually referred as transdermal iontophoresis [6].
The technique requires the application of two oppositely charged electrode across skin so as to drive drug molecule with similar charge as electrode by repulsion [7]. The medicated or transdermal patch is a unique device which is meant to be placed over treatment surface with intention to deliver medication into blood stream and to provide advantage of controlled release of drug for longer period of time and marked reduction in dosing of potent drug. The applied patch can be either porous or reservoir type of membrane [8]. Local anaesthetic when applied triggers the chain of sequences initiating many actions on muscles and nerves with marked increase or decrease in circulation to remove drug from site of action. The total plasma level (of local anaesthetic) can be lowered by reversing blood flow patterns to decrease local body distribution of drug [9]. The vasomotor effects of the Lignocaine are concentration dependent.
The Epinephrine, a hormone, neurotransmitter and a sympathomimetic amine interacts with adrenergic receptors (while action varies from tissue type and expression of adrenergic receptors) shows unequivocal relationship between concentration and pain after surgery. The hormone when added exogenously induces pain but prolongs the action (via vasoconstriction effect) results in perfusion of tissue and oxygen availability [10]. Clonidine, a centrally acting α2 adrenergic agonist and imidazoline receptor agonist can be used to ease withdrawal symptoms of long term use of narcotics, alcohol, nicotine etc. It also alleviate opioid withdrawal symptoms by smoothening sympathetic nerve response as well as reducing sweating, hot and cold flushes [11,12]. It has been studied that the addition of Clonidine to local anesthetics in peripheral nerve block prolongs block duration as well as postoperative analgesia. Clonidine shows local vasoconstriction effect by postsynaptic adrenergic receptor activation which leads to delayed systemic absorption of the local anesthetic and thus to prolongation of the local anesthetic effect.
Lignocaine or Lidocaine or Xylocaine popular local anesthetic agent titled under the class of 1b-antiarrhythmatic drug. Along with its local application it can also be used intravenously as dental anesthetic and for minor surgery [13] due to its rapid onset of action (between 20 seconds to 1 minute) and intermediate duration of efficacy (5-30 min.). Therefore it has been used in mouth ulcer, for infiltration block and surface anesthesia. Lignocaine is absorbed rapidly following topical administration to mucous membranes [14]. The rate and extent of the absorption depends on various factors such as concentration, viscosity of the agent, duration of exposure, specific site of application. Lignocaine has been used to treat ulcers due to its excellent local anesthetic effect that leads to relieve the pain [14]. Iontophoretic patch Companion 80-24 h Iontophoresis Patch. IOMED Companion 80 Electrodes are an integrated solution for providing fast, easily administered, long lasting iontophoresis drug delivery. An on-board battery and low current, long duration approach allows the patch to deliver drugs without the need for any additional equipment. Integrated electrode design and on-board battery means not having to deal with stationary iontophoresis equipment. Superior design and breathable, flexible material allows twenty four hours of comfortable wear. Easy application and nonirritating, hypoallergenic adhesive allow fast, hassle-free treatment. The purpose of conduction of the present study was to broadcast the effect of Epinephrine and Clonidine on Local anaesthetic activity of Lignocaine. Gaumann et al. had proposed that addition of Clonidine to the solution of Lignocaine for the peripheral nerve block prolongs the anaelgesic duration and action [15]. Lignocaine when combines with epinephrine shows intensifying action due to restriction of spread of nerve impulse [16].
Materials and Methods
Lignocaine and Epinephrine and Clonidine were generously gifted by Shouguang Fukang Pharmacy Factory (Shandong, China). Polymers of Hydroxy propyl methyl cellulose (HPMC; 261.45 g/mol and viscosity is 2,600-5,600 cP, 2 % in H2O.) and (EC; 454.513 g/mol and viscosity is 10 cP, 5% in toluene/ ethanol 80:20) materials were purchased from Sigma Aldrich; USA. All other chemical agents and materials were of analytical grade and used as received.
Preparation of patch enclosing local anaesthetic with epinephrine and clonidine
The polymers of HPMC and EC were accurately weighed and dissolved in ethanol to prepare 5% solution of Polymer (Polymeric solution). The drug solutions (Lignocaine +Epinephrine) were prepared by dissolving the predetermined amount of drugs in methanolic solution (solution A). Another drug solution (Lignocaine+Clonidine) was prepared using same solvent with same strength (solution B).
Thus prepared polymeric solution was poured in glass petri dish (whose diameter was previously measured) to form the backing membrane of the patch. The dish was then dried in hot air oven at 50°C. To the drug solution (Solution A) plasticizer of glycerol was added and thus prepared solution was poured onto the polymeric backing membrane. The prepared plate was dried at 55°C for 4-5 h. After drying the patch was removed and cut into small pieces and evaluated for various parameters [15]. The same procedure was followed for the solution B i.e. for drug Clonidine. The Blank sample was prepared i.e. only 2 % Lignocaine only.
Preparation of patch enclosing electrolyte solution
The electrolyte reservoir was prepared by same method as described above which contains glycerin, sodium chloride, preservatives (2-phenoxyethanol, methyl-,ethyl-, propyl-,butyl-,and isobutyl-p-hydroxybenzoate) and monobasic sodium phosphate.
Evaluation of patches
The clarity and smoothness of the patches was observed visually. Micrometer screw gauge was used to check thickness at 5 different locations whose average was taken. The weight of patches from each batch was measured and average weight was determined. Number of times the patch can be folded at same place without cracking shows the folding endurance. The strength of the patch was determined by using the folding endurance test which was performed by repeatedly folding the patch at the same place until it broke. Tensile strength of the patch was determined with Universal strength testing apparatus.
The test for content uniformity involves socking of 5 patches in 15 ml methanolic solution which was filter through 0.3 micron filter paper to remove undissolved polymeric residue. HPLC system with UV-Vis detector (SPD-10 A) with C-18 reverse phase column was used. A filtrate of approx 10 μL was injected using mobile phase of acetonitrile and phosphate buffer pH 7.4 (95:5 V/V). The flow rate was 1.0 ml/min with detection at 220 nm.
Percentage moisture loss was determined by keeping patches in a desiccators previously saturated with anhydrous calcium chloride the patches were weighed (W1) for 5-6 days. After completion of period they were taken out to re-weight (W2). The difference between W1 and W2 with respect to W1 will tell us about the % moisture loss. To determine % moisture absorption the same procedure was repeated with vaccume desiccators.
In vitro drug release was calculated with the help of modified glass diffusion cell. At the one end of which cellophane membrane (previously soaked in phosphate buffer pH 7.5 for 24 h) was attached. The temperature was maintained at 37°C by circulating water in water bath at 60 rpm on magnetic stirrer. Donor and receptor compartments were adjusted such that the membrane just touches the surface of receptor medium. The patches were cut into the dimension of 1 cm2 and placed on cellophane membrane. The aliquot of 5 ml was withdrawn at specific intervals and simultaneously replaced with fresh buffer to maintain sink condition. The sample thus obtained was fed into HPLC system using UV detector at 240 nm, (C 18 column/Acetonitrile: Phosphate buffer 95:5 V/V). The flow rate of 1 ml/min was maintained [17].
The protocol for the present study was approved by human ethical committee of Department of Anesthesiology, Beijing Shijitan affiliated hospital of the capital medical University, Beijing 100038, China (Protocol no: Clin/BSH/0045/Anes). The nature, structure and intention of the study and written informed consent was obtained from volunteers. The trial was a controlled, randomized containing group of 20 subjects in each group (n=20) where Male: Female ratio is equal. The groups were named as Group A or Epinephrine group, Group B or Clonidine group, Group C or Blank group. Patients were allowed to take neither premedication nor additional medicines. All Medications were banned in the total period of trial for the drug who interfere with blood pressure, heart rate or with anaesthetic activity. On arrival into experimental room the subjects were equipped with standard medical devices such as electrocardiogram, automatic blood pressure device. The structure of the entire study makes three groups of subjects, each containing 20 no’s (10 M:10 F). At the time of termination of the study total 59 subjects were examined. All the tests and trials were successfully carried out except in one subject from clonidine group who was excluded during study.
Application of Iomed system and patches to volunteers
After application of the device to the respective groups and on arrival to the experimental room they were equipped with electrocardiogram, automated blood pressure machines and closely monitored for the B.P. and H.R. The patients responses for the specific end points were also recorded. The IP was applied for 10 min only.
End points measurements
The observation of end point and measuring the intensity of the same was done at interval of 0, 5,10,15,20 and 30 minutes after the treatment. The physical stimuli like sensory block, warm, cool, pain intensity, Patient sedation, Plasma drug concentration were considered as prime parameters. A 27 gauge needle was used to determine sensory block/depth of anesthesia. For assay purpose the samples were collected at the interval of 0, 10, 30 minutes [18]. The test for sensory block was performed by/under supervision of trained anaesthesiologist. The ulnar, radial and musculoculaneous nerves were identified and pricked with help of 27 guage top pole insulated needle injected at anaesthetized site in the blindfolded subjects. The response of the patient was evaluated for every 5-10 min until 30 min or until complete recovery whichever is early. Three pointer scale (0, Normal sensation; 1, blunted sensation; 2, No perception) was used for evaluation of complete block of sensory and/or motor nerves [18].
The cool sensation and warm sensation was measured by the stimulus produced using meander electrode (which consist of electrodes of alternating polarity with changeable temperature by 1.0°C/s). The lowest produced stimuli was measured first followed by highest. The switch was surrendered to the patient so he/she can press it when the first sensation of warmth or cold was felt. Reversing the switch will also reverse the temperature to neutral i.e. 32°C. Similarly the Thermal Sensory Analyzer was used for warm pain sensation but the end point here is pain instead of temperature. The 10 pointer scale or VAS scale (visual analogue scale) was used here having 10 steps as 0, No pain to 10 as worst pain [18-20]. The skin was examined superficially for any visible signs of swelling, itchiness, redness etc. The degree of Patient sedation was determine using the 5-point scale indicating 0-4 (where 0- awaken; 1-drowsy; 2-dozing; 3-asleep; 4,awake only when arouse).
Blood samples from the subjects were collected at different time intervals (before and after treatment) so as to determine plasma level concentration of Lignocaine. The detection of the local anaesthetic drug in the blood was done by Fluorescent technique having sensitivity of about 0.45 μmol/L. Rowland et al. has proposed that rate of elimination of Lignocaine follows first order kinetics [21]. Statistical analysis of data was done using unpaired t-test. ANOVA of sedation and/or pain was performed by Mann-Whitney U-test.
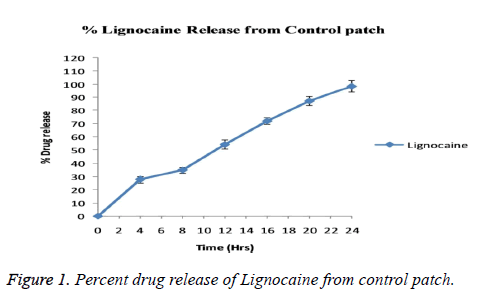
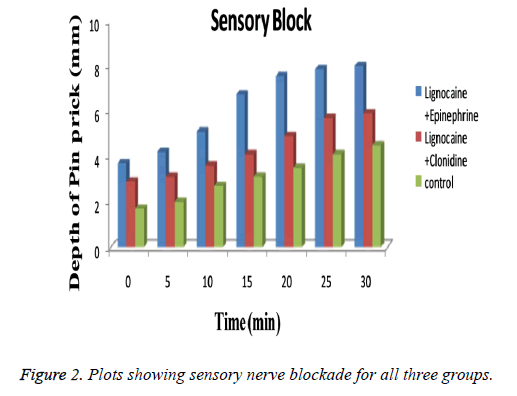
Blood pressure and heart rate of the patients were continuously monitored through the operation and also recorded posttreatment at the same time as the pain scores. No significant changes in blood pressure and/or heart rate were found during entire test procedure. The values were not differ from that in baseline values. As summarized in Table 1 groups used in the study bears similarity with respect to age (p=0.58), body weight (p=0.69), height (p=0.71) etc. The technique used for application of device is also same for both groups. After observing the results obtained from patch evaluation Table 2 it can be stated that the method used for preparation i.e. solvent evaporation was successful. The smooth and uniform surfaced patches were obtained with thickness in the range of 0.04 ± 0.007 to 0.06 ± 0.12 mm. The tensile strength of the patches were found to be 0.54 to 0.61 kg/cm2 having average weight of 0.17 ± 0.008 gm. Moisture content was found to be 1.19 ± 0.32% with folding endurance of 151.12 ± 0.4. The patches were found to release the drug in-vitro in phosphate buffer as 89.54 ± 0.45. The release profile of drug from the patch is shown in Figure 1. The depth of sedation or blockade of sensory or motor nerves was found to be indifferent for three groups as shown in Figure 2. The progression of the test was stopped immediately after experienced of the pain. Maximal Pain score was found to be higher in subjects of group B or in Clonidine group (range 1-8) than in those treated with Epinephrine (range 0-7). The large amount of pain was sensed by those in group C or control group (range 2-9). Some patients in Epinephrine group were found free from pain sensation (VAS scale score 0) till 10 h. The time course of sedation in terms of sedation score is illustrated in Table 3.
| Characteristic | Epinephrine group (A) | Clonidine group (B) | Control group (C) |
|---|---|---|---|
| M/F | 10/10 | 10/10 | 10/10 |
| Age | 39 ± 17 | 35 ± 19 | 41 ± 11 |
| Height (cm) | 182 ± 17 | 179 ± 19 | 171 ± 21 |
| Weight (kg) | 78 ± 9 | 81 ± 12 | 79 ± 17 |
Table 1. Clinical details of 60 Subjects involved in the trial.
| Batch | Appearance | Drug content uniformity (%) | Folding endurance | In-vitro release | Tensile strength (Kg/cm2) |
|---|---|---|---|---|---|
| A | T | 0.04 ± 0.007 | 151.12 ± 0.4 | 89.54 ± 0.45 | 0.54 ± 0.14 |
| B | S | 0.06 ± 0.12 | 142.76 ± 0.8 | 91.40 ± 0.51 | 0.61 ± 0.21 |
| C | S | 0.049 ± 0.18 | 134.08 ± 0.6 | 87.21 ± 0.23 | 0.57 ± 0.18 |
Table 2. Evaluation and physical characterization of patches.
| Patient group | Time after axillary block (min) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | |
| Epinephrine Group | 0 (0) |
0 (0-3) |
0 (0-3) |
0 (0-3) |
0 (0-2) |
0 (0-2) |
0 (0-2) |
| Clonidine Group | 0 (0) |
0 (0-1) |
1 (0-1) |
1 (0-2) |
1 (0-2) |
1 (0-3) |
0 (0-3) |
Table 3. Sedation score ranges treated groups.
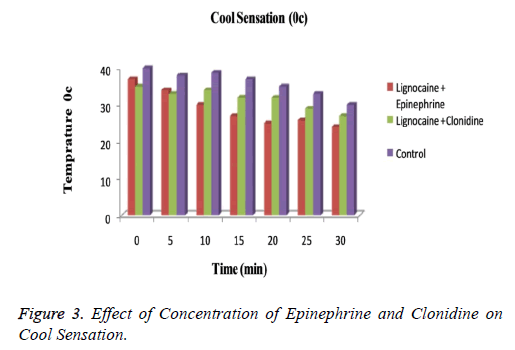
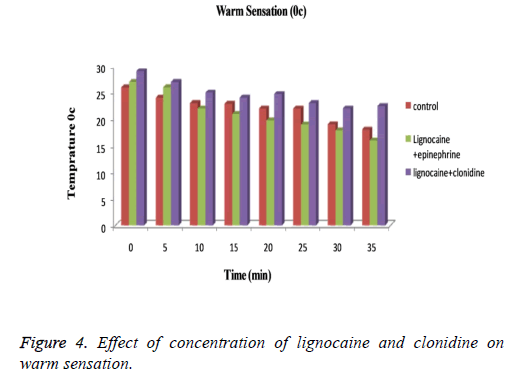
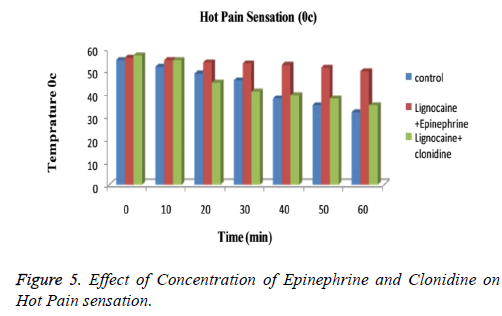
The subjects treated with Clonidine (range 0-4) had showed marked sedation as compared to those with Epinephrine (range 0-2). Figure 3 explained that for cool sensation at 0 h there were no sign of significant difference between all three groups. The difference starts to increase as the time passes. The Lignocaine along with Epinephrine shows noticeable ability to overcome cool sensation when compared to other two formulations. As per denotation in Figure 4 one can say that despite of initial similarity, Epinephrine enhance the ability of Lignocaine to combat warm sensation over Clonidine and control formulation. Figure 5 depicts the effect of epinephrine and lignocaine concentration on hot pain. The baseline values for all the groups are almost same. As the time passes from 0 min to 30 min the hot pain sensation decreases sharply for Epinephrine while it shows less reduction in Clonidine group.
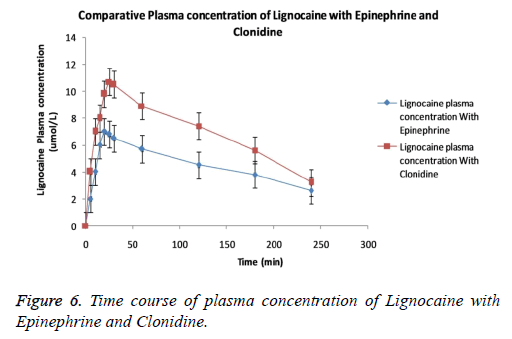
The curve lines in the Figure 6 specifies that co-administration of Clonidine and/or Epinephrine to Lignocaine remarkably produces difference in plasma concentration of Lignocaine. The curve line in the graph shows that plasma concentration of Lignocaine with Clonidine is readily attained higher than Epinephrine. The reason underlying may be the rapid absorption of Lignocaine in presence of Clonidine. All three groups showed no or minimal difference between elimination half life and area under curve at end of experiment. Mild type of irritation persisting only for 2-5 min was observed in few patients entering the trial. No patient involved report swelling, itching, edema.
Discussions
The current study emphasise on the local anaesthetic activity enhancing effect of epinephrine and Clonidine on Lignocaine. Epinephrine also known as adrenaline alleviates pain if given exogenously. But the drug is reported to enhance the anaesthetic activity of Lignocaine (via complex set of biochemical or cellular reactions) when co-administered. These receptor mediated dynamics is thought to be the reason of soothing action of epinephrine. Clonidine, an anti-hypertensive (α2-adrenergic agonist) drug when given in combination has stated to enhance the analgesic effect of Lignocaine. Tigka et al. has proved the influence of Clonidine on the Lignocaine with help of protein binding in Rats.
The short duration of action of plain Lignocaine provides better comparison effect for other drugs. The reason behind boycotting the longer acting anaesthetic is prolongation of time period which would unnecessary lengthens time involved in trial. About 150 μg of clonidine, 200 μg (1:200,000) of epinephrine was selected by analyzing the previous reports which certifies them as optimal for local vasoconstriction effects [22]. The references used above provide sufficient assurance that 150 μg of clonidine and 200 μg (1:200,000) of epinephrine provides identical pharmacodynamic effect on Lignocaine [23]. The difference in the peak plasma concentration of Lignocaine with Clonidine and Epinephrine may be due to different mechanism of action. Clonidine shows increase in absorption of Lignocaine when compared to Epinephrine. The reason behind enhanced absorption of Lignocaine can be predicted as direct action on nerve rather than vasoconstriction. But advantage of administering Lignocaine with Epinephrine is less chances of systemic toxicity due to delayed absorption [23] in comparison to clonidine who’s produces small safety margin with regard to local anesthetic.
The overall observations also shows that the responses obtained were same initially for all the three groups but those with Lignocaine and Epinephrine feels greater level of anesthesia than other two. Wallace et al. had shown that mylonated fibres are more sensitive to local anaesthetic blockade than un-mylinated fibres [23]. By carefully observing the reading one can say that maximal pain score was higher in patients administered with Lignocaine with Clonidine as compared to those with Lignocaine and Epinephrine.
Conclusion
The study presented above concludes that co-administration of 150 μg of clonidine and/or 200 μg of epinephrine can be used to enhance the local anaesthetic effect of Lignocaine. Analgesic effect produced was more for prominent for Epinephrine than for Clonidine. Despite Clonidine is not as efficacious for producing analgesia as epinephrine, it can be used as alternative in patients allergic to epinephrine.
References
- Tyle P. Iontophoretic devices for drug delivery. American Cyanamid Company, New Jersy,1986.
- Sachan R, Bajpai M. Transdermal drug delivery system: a review. Int J Res Dev Pharm Life Sci 2013; 3: 748-765.
- Dedakia A, Matholiya C, KoyaniV, Bhimani D. Three generations: pimary, secondary and tertiary generations of transdermal drug delivery systems: A review. Int J Pharm Sci Res 2015.
- Shingade GM, Aamer Q, Sabale PM, Grampurohit ND, Gadhave MV. Review on: recent trend on transdermal drug delivery system. J Drug Deliv Ther 2012; 1: 66-75.
- Prausnitz MR. Reversible skin permeabilization for transdermal delivery of macromolecules. Crit Rev Therap Drug Carrier Syst 1997; 14: 455-483.
- Hofmann GA, Rustrum WV, Suder KS. Electro-incorporation of microcarriers as a method for transdermal delivery of large molecules. Bioelectrochem Bioenerg 1995; 38: 209-222.
- Wallace SM, Ridgeway B, Jun E. Topical delivery of Lodocaine in healthy volunteers by Electroporation, Electroincorporation, or Iontoporesis: An evaluation of skin Anesthesia. Reg Anesth Pain Med 2001; 26: 229-238.
- Patel D, Chaudhary SA. Transdermal Drug Delivery System: A Review. Pharma Innovat 2012; 1: 66-75.
- https://www.recallguide.org/drug/lidosite/
- Rawat S, Vengurlekar S, Rakesh B, Jain S, Srikarti G. Transdermal delivery by iontophoresis. Indian J Pharm Sci 2008; 70: 5-10.
- https://en.wikipedia.org/wiki/Clonidine
- Fitzgerald PJ. Elevated Norepinephrine may be a Unifying Etiological Factor in the Abuse of a Broad Range of Substances: Alcohol, Nicotine, Marijuana, Heroin, Cocaine, and Caffeine. Subst Abuse 2013; 7: 171-183.
- Ahmad J, Andrabi S, Rathore M. Comparison of topical glyceryl trinitrate with lignocaine ointment for treatment of anal fissure: A randomised controlled trial. Int J Surgery 2007; 5: 429-432.
- Donatella M, Fulvia B, Tito P. Effects of lignocaine blockades and kainic acid lesions in the Bötzinger complex on spontaneous expiratory activity and cough reflex responses in the rabbit. Neurosci Lettr 2002; 332: 175-179.
- Gaumann D, Forster M, Griessen M, Habre W, Pohsot O. Comparison between clonidine and epinephrine admixture to lidocaine in brachial plexus block. Anesth Analg 1992.
- http://www.drugsupdate.com/generic/view/134/Epinephrine-+-Lidocaine
- Demiana I, Nesseem SF, Eid SS, El-Houseny. Development of novel transdermal self-adhesive films for tenoxicam, an anti-inflammatory drug. Life Sciences 2011; 89: 430-438.
- http://www.accessdata.fda.gov/scripts/CDER/dissolution/dsp_SearchResults_Dissolutions.cfm
- Catherine J, Sinnott LP. On the mechanism by which epinephrine potentiates lidocaine’s peripheral nerve block. Anesthesiology 2003; 98: 181-188.
- Yarnitsky D, Ochoa JL. Warm and cold specific somatosensory systems. Psychophysical thresholds, reaction times and peripheral conduction velocities. Brain 1991; 14: 1819-1826.
- Gasser HS, Erlanger J. The role of fiber size in the establishment of nerve block by pressure or cocaine. Am J Physiol 1929; 88: 581-591.
- Gissen AJ, Covino BG, Gregust J. Differential sensitivities of mammalian nerve fibers to local anesthetic agents. Anesthesiology 1985; 53: 467-474.