ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2005) Volume 16, Issue 1
Isolation of Excretory Secretory Protein 6 kda antigen (ES-6) and its seroreactivity in patients with different stages of pulmonary tuberculosis and healthy household contacts
Jamnalal Bajaj Tropical Disease Research Centre, India
1Department of Biochemistry, Mahatma Gandhi Institute of Medical Sciences, Sevagram—442102, Wardha, Maharashtra, India
- *Corresponding Author:
- Dr. B.C. Harinath
Jamnalal Bajaj Tropical Disease Research Centre
Department of Biochemistry, Mahatma Gandhi Institute of Medical
Sciences Sevagram 442102, Wardha, Maharashtra, India
Phone/ Fax: +91-7152 284038
E-mail: jbtdrc_wda ( at ) sancharnet.in
Accepted date: December 14 2004
An Excretory Secretory protein antigen of 6 kDa (ES-6) was isolated from Mycobacterium tuberculosis H37Ra culture filtrate by gel filtration using fast protein liquid chromatography. Seroreactivity of ES-6 antigen was compared with earlier reported diagnostically useful ES-31 and ES-43 antigens at different stage of pulmonary tuberculosis and in household contacts of the patients. The ES-31 and ES-43 antigens showed good immune response in chronic and relapse cases respectively while ES-6 antigen has shown comparatively low immune response in these cases. However ES-6 showed in-creased seroreactivity in household contacts of pulmonary tuberculosis patients. These results suggest the heterogeneous responses of antigens in different disease conditions and immune response to ES-6 antigen may be associated with latent infection for predicting active disease in course of time, as observed in the follow up of these individuals.
Keywords
Excretory secretory antigen, ES-6, ELISA, Humoral response, Tuberculosis disease, Household contacts.
Introduction
Tuberculosis is still prevalent in developing countries and is the leading killer disease. Co-infection with human immunodefi-ciency virus (HIV) and Mycobacterium tuberculosis increases the risk of developing tuberculosis [1]. In addition, multi drug resistant tuberculosis is rapidly spreading and more efficient methods to prevent and control the disease are urgently needed. A major obstacle to effective tuberculosis control and its eventual elimination is the inability to accurately identify the subset of persons who are infected with tuberculosis. Serodiagnosis of tuberculosis is considerably simpler and cheaper and hence extensively investigated [2,3]. Lack of reliable diagnostic test for all cases of tuberculosis may be due to the substantial and unexplained heterogeneity of humoral response to different antigens of M. tuberculosis [4,5]. Hence it will be of interest to analyze the humoral immune response to different excretory secretory (ES) antigens of M. tuberculosis among the patients with active disease and healthy contacts with latent infection who are at risk of getting full blown disease. Hence in this study an attempt was made to investigate serological response using different M. tuberculosis ES-31, ES-43 and another low molecu-lar weight ES-6 antigen.
Materials and Methods
Subjects
Blood samples were collected from confirmed pulmonary tuber-culosis patients attending District Tuberculosis Centre, Wardha and Kasturba Hospital, Sevagram. These cases were further categorized into fresh, chronic and relapse, following Revised National Tuberculosis Control Programme.
Fresh cases (n=25)
A new case who has never had treatment for tuberculosis or who is freshly diagnosed as tuberculosis case and taken antitubercular drug for less than four weeks. Patients in this category were considered to have sign and symptoms of tuberculosis for less than four weeks.
Relapse case (n=30)
A patient who has been declared cured of any form of tubercu-losis in past by a physician after one full course of chemotherapy and has again become sputum smear positive.
Chronic case (n=25)
A patient who remained or became again smear positive after completing a fully supervised re-treatment regimen.
Blood samples were also collected from 20 healthy individuals with no history of contact with tuberculosis patients of this local-ity served as healthy control. Blood samples were also collected from 10 contact cases with history of person-to-person contact. These includes Household contacts persons living for > 6 months in the same house with the patient. Sera were sepa-rated and stored at –20°C with 0.1% sodium azide.
Isolation of M.tb H37Ra excretory-secretory low molecular weight 6 kDa (ES-6) antigen
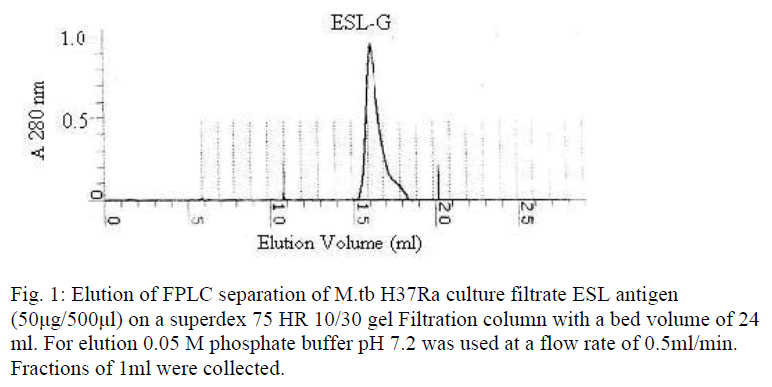
The excretory-secretory low molecular weight (<10 to12 kDa) antigen (ESL-Ag) was prepared from M.tb H37Ra ten days spent culture (Sauton medium) of exponentially growing tubercle ba-cilli. The bacilli were separated from medium by filtration through Whatman 3 filter paper. The culture fluid was freed of bacilli by filtration through Seitz filter. The culture filtrate was passed through ultra filtration unit (Millipore, USA) using a membrane of 10-12 kDa molecular weight cut off. The filtrate obtained was further concentrated using Amicon ultra filtration stirred cells (Millipore, USA) using 1 kDa cutoff YM1 membrane. The concentrated filtrate was dialysed extensively against 0.01M PBS (pH 7.2) using same unit. Further ESL-Ag fraction was fractionated by fast protein liquid chromatography using Superdex HR (High Resolution) 10/30 gel filtration column (Pharmacia Biotech, Sweden) following manufacturer’s instruc-tions. M.tb ESL Ag (50μg/500μl) was applied and eluted using 0.05 M PBS pH 7.2 at a flow rate of 0.5 ml/min and monitored at 280 nm. Fractions of 1ml volume were collected and the frac-tions of protein peak obtained were pooled and checked for antigenic activity by indirect ELISA using pooled tuberculosis positive and negative control sera.
M.tb ES-31 antigen is isolated from M. tb crude culture filtrate ES antigen by affinity chromatography using affinity purified anti ES-31 antibody coupled Sepharose 4B column as described earlier [6]. M.tb H37Ra ES-43 antigen was isolated from 50% ammonium sulphate solubalized portion of excretory-secretory antigen, followed by further fractionation on Sodium Dodecyl Sulphate Poly Acrylamide Gel Electrophoresis (SDS-PAGE). Gel eluted from 6th fraction purified by fast protein liquid chroma-tography (FPLC) using Resourse ‘Q’ 1ml anion exchange col-umn as described earlier [7].
Indirect ELISA
Indirect penicillinase ELISA was performed as described by Nair et al [8], to evaluate the reactivity of FPLC fraction and purified antigens for detection of tuberculous IgG antibodies in human sera. Five μl of optimally diluted antigen [ES-31 or ES-43 (0.2μg/ml)], and serial ten fold dilutions of FPLC fractions (ES-6) with starting concentrations of 200μg/ml was applied on cellu-lose acetate membrane (CAM) square fixed to plastic strip, op-timally diluted human sera (1:600) and antihuman IgG penicilli-nase conjugate (1:1000) were used in this assay. The sera showing complete decolorization of blue colour (Starch Iodine Penicillin ‘V’) substrate at least 5 minutes earlier than the negative control denoted a positive reaction.
Results
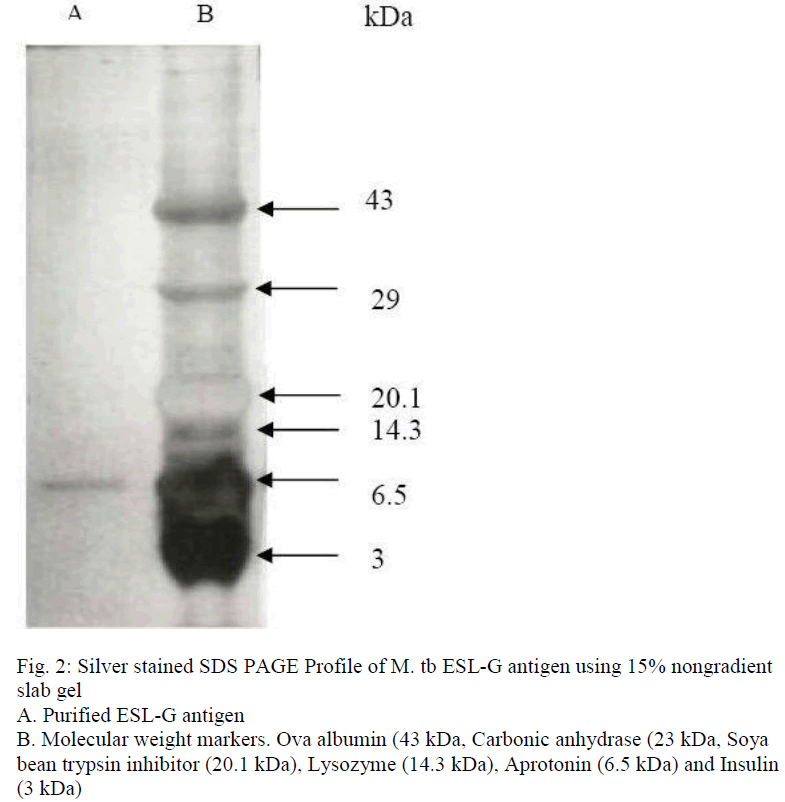
Fractionation of M.tb ESL antigen by FPLC, using superdex 75 HR 10/30 gel filtration column chromatography, resolved into a single peak (fig.1). The fractions under the peak were pooled and designated as ESL-G. When analysed by indirect ELISA, the fraction showed higher antigenic activity (at antigen concen-tration of 0.2 μg/ml i.e. 1ng/stick). ESL-G protein on separation by SDS-PAGE using 15% nongradient gel and silver staining showed a single protein band corresponding to a molecular weight of approximately 6 kDa (fig. 2) and was labelled as ES-6 antigen.
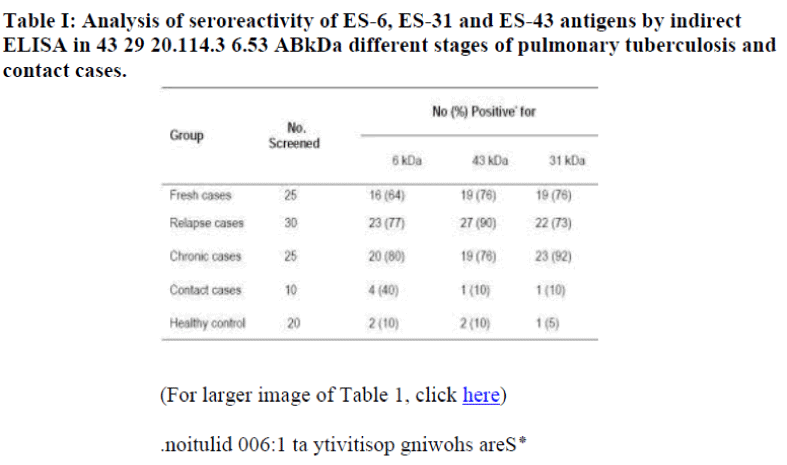
The seroreactivity of ES-6 antigen was compared with ES-31 and ES-43 antigens for detection of tuberculous IgG antibody by indirect penicillinase ELISA in different stages of pulmonary tuberculosis cases, household contacts and in healthy control individuals. Using ES-6 antigen only 64% (16/25) of fresh cases showed positive reaction compared to 76% (19/25) using ES-31 and ES-43 antigens. In chronic cases 80% (20/25) patients showed positive reaction for ES-6 antigen compared to 92% (23 of 25) for ES-31 and 76% (19/25) for ES-43 antigen fractions. While out of 30 relapse cases, 27 (90%) showed positive reac-tion to ES-43 antigen compared to 23 (77%) and 22 (73%) for ES-6, ES-31 antigens respectively. (Table I). On the other hand 40% (4 of 10) of household contact showed positivity to ES-6 antigen compared to 10% (1/10) for ES-31 and ES-43 antigen. Two of 20(10%) healthy control subjects showed positivity to ES-6 antigen and ES-43 antigen, while 1 of 20 (5%) showed positivity to ES-31 antigen.
The Geometric mean titre (GMT) of tuberculous IgG antibodies in different groups of sera was analysed. The GMT of tubercu-lous IgG antibody against ES-6 antigen in all diseased cases (GMT 519, 613 and 634 in fresh, relapse and chronic cases respectively) was lower compared to ES-31 (GMT 523, 645 and 904 respectively) and ES-43 (GMT 539, 1039 and 753 respectively). In household contacts the GMT was higher (GMT 487) against ES-6 compared to that of ES-31 (GMT 280) and ES-43 (GMT 300). While in healthy control GMT of tuberculous IgG antibody were 191, 178 and 185 for ES-6, ES-31 and ES-43 antigens respectively.
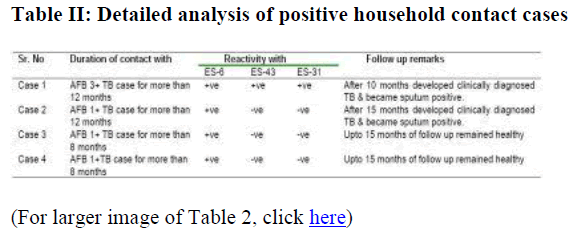
Among the contact cases, which has shown positivity to ES-6 antigen, case 1 had history of close contact of AFB 3+ Tuberculosis patient and case 2,3 & 4 had the contact with AFB 1+ tuberculosis patient for duration of 12 to 8 months (Table II). Case 1 which had shown reactivity to all these antigens, developed clinically diagnosed tuberculosis after 10 months of follow up and became sputum positive. Case 2 which had shown positivity to ES-6 antigen only, developed clinically diagnosed tuberculosis, at 15 months of follow up and became sputum positive, while cases 3 and 4 remained healthy upto 15 months at following.
Discussion
Culture filtrate contains large number of excretory-secretory proteins and few have been purified and studied in detail. In the view of current alarming increase in the incidence of tuberculosis all over the world, there is now more than ever, an urgent need for research in identification of antigens of diagnostic and immunomonitoring interest in tuberculosis. It has been suggested that surface proteins and proteins actively secreted by Mycobacterium tuberculosis are important targets of immune system during the early phase of an infection. In the present study a low molecular weight ES-6 antigen was isolated from Mycobacterium tuberculosis H37Ra culture filtrate and it’s reactivity was compared with earlier reported diagnostically useful ES-31 and ES-43 antigen which had shown more reactivity in chronic and relapse cases respectively [7]. Further the reactivities of these antigens were compared in household contacts which are at high risk of having sub clinical infection due to continuous exposure to tuberculosis patients and may develop disease and hence need to be diagnosed early for effective tuberculosis control and arrest disease development.
The ES-6 antigen has shown comparatively less reactivity in fresh, relapse and chronic cases compared to ES-31 and ES-43 antigens. The GMT of tuberculous IgG antibody was lower for ES-6 antigen fraction in these groups, compared to ES-31 and ES-43 antigen fractions. However 40% household contact cases showed reactivity to ES-6 antigen compared to 10% to ES-31 and ES-43 antigens. The GMT of tuberculous IgG antibody to ES-6 antigen was higher in these cases compared to ES-31 and ES-43 antigens. Thus results shows heterogenecity of humoral immune response to different antigens of Mycobacterium tuber-culosis in the patients with different stages of disease, as observed in our earlier studies [7,8] which are in agreement with other studies [5,9]. Further it can be speculated that positivity to ES-6 antigen may be more associated with sub clinical or latent infection. Andersen et al [10] has shown proteins from low molecular weight regions 4-11 kDa and 26-35 kDa to possess marked stimulatory properties, out of which prominent immuno-stimulatory Tcell antigen ESAT-6 has been characterized to Mycobacterium tuberculosis complex specific. Cell mediated response to ESAT-6 measured by lymphocyte proliferation or gamma interferon production has been studied in active tuberculosis patients and healthy household contact (often higher levels than tuberculosis patients) and general population samples in endemic countries [11-14]. However few studies have been reported on humoral response to ESAT-6 antigen. Silva et al [9] found that humoral response to 38 kDa antigen was correlated with active tuberculosis disease while ESAT-6 and 14 kDa correlated with inactive rather than active disease and may be associated with reactivation of disease.
Two earlier studies also have suggested that humoral response is predictive of tuberculosis disease. In one humoral response to Mycobacterium tuberculosis antigens was increased in patients with inadequate cell mediated response to the same antigens [15] and in other 40% of patients with a strong humoral re-sponse to glycolipid antigen developed active tuberculosis [16] The healthy control subjects which has shown reactivity in our study may have unrecognized exposure to M. tuberculosis.
In this study on follow up of four contact cases which had shown positivity to ES-6 antigen, the case 1, the contact case of AFB 3+ TB patient for more than 12 months developed clinically di-agnosed tuberculosis and became sputum positive after 10 months. The case 2, the contact case of AFB 1+ TB patient for more than 12 months duration developed clinically diagnosed TB, and became sputum positive after 15 months of follow up. While the other cases remained healthy up to 15 months of follow up. Thus probability of acquisition of infection is not only related to the potential infectiousness of the putative source but also dependent on the duration of exposure as observed in these cases. The existing protocols recommended for contact tracing investigation procedures also emphasize on these two factors [17]. Thus it can be concluded that increased humoral response to ES-6 antigen among contact cases may be a predictive marker of future disease development and needs contin-ued follow up of these cases. Contact investigation is being given importance in control of tuberculosis and thus analysis of serological response to different M. tuberculosis antigens may aid in developing efficient investigation procedures for contacts.
Acknowledgement
We thank Shri Dhirubhai Mehta, President, Kasturba Health Society and Dean, Dr. (Mrs) P. Narang for their keen interest and encouragement for this study. This work is partly funded by grant from CSIR. Our thanks are due to Dr. Kalsait, District Tuberculosis Officer, Dr. Hiwrale, Medical Officer, Civil Hospital, Wardha and their staff for extending cooperation in follow up patients. Technical assistance of Mrs. S. Ingole is appreciated.
References
- Kochi A. Tuberculosis distribution, risk factors, mortality. Immunobiol 1994; 191: 325-326.
- Daniel T. Immunodiagnosis of tuberculosis. In: Rom WM, Garay S, eds. Tuberculosis. Boston MA: Little, Brown and Co. 1996: 223-231.
- Daniel TM, Debanne SM. The serodiagnosis of Tuberculosis and other mycobacterial diseases by enzyme linked immunosorbent assay. Am Rev Respir Dis 1987; 135: 1137-1151.
- Lyaschenko K, colangeli R, Houde M, Al Jahdali H, Menzies D, Gennaro MI. Heterogenous antibody responses in tuberculosis. Infect Immun 1998; 66: 3936- 3940.
- Samanich KM, Keen MA, Vissa VD, Harder JD, Spencer JS, Belisle JT, Zolla Pazner S, Laal S. Serodiagnostic potential of culture filtrate antigen of Mycobacterium tuberculosis. Clin Diagn lab Immunol 2000; 7: 662-668.
- Nair ER, Banerjee S, Reddy MVR, Kumar S, Harinath BC. Isolation of Mycobacterium tuberculosis 31 kDa antigen protein of diagnostic interest from culture filtrate using anti ES-31 antibody by affinity chromatography. Ind J Clin Biochem 2001; 16: 132-135.
- Gupta S, Shende N, Kumar S and Harinath BC. Antibody response to M.tb H37Ra excretory secretory ES-43 and ES-31 antigens at different stages of pulmonary tuberculosis. Bio-medical Research; 2004; 15: 76-79
- Nair ER, Banerjee S, Kumar S, Reddy MVR, Harinath BC. Purification and characterization of a 31 kDa mycobacterial excretory secretory antigenic protein with a diagnostic poten-tial in pulmonary tuberculosis. Ind J Chest Dis Allied Sci; 2001; 43: 81-90.
- Silva VMC, Kanaujia G, Gennaro ML, Manzies D. Factors associated with humoral response to ESAT-6, 38 kDa and 14 kDa in patients with a spectrum of tuberculosis. Int J Tuber Lung Dis 2003; 7: 478-484.
- Andersen P, Askgaard D, Gottschau A, Bennedsen J, Nagai S, Heron I. Identification of immunodominant antigens during infection with Mycobacterium tuberculosis. Scand J Immunol 1992; 36: 823-831.
- Lalvani A, Pathan AA, Mcshane A, Wilkinson RJ,Latif M, Conlon CP, Pasvol G,Hill AV. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen specific Tcells. Am J Respir Crit Care Med 2001; 163: 824-828.
- Pathan AA, Wilkinson KA, Klenerman P, MacShane H, Davidson RN, Pasvol G. Direct ex vivo analysis of antigen specific IFNY secreting CD4 T cells in Mycobacterium tuber-culosis infected individuals; associated with clinical disease state and effect of treatment. J Immunol 2001; 167: 5217-5225.
- Vekemans J. Lienhardt C, Sillah JS, Wheeler JG, Lahai GP, Doherty MT, Corrah T,Andersen P,McAdam KP, Marchant A. Tuberculosis contacts but not patients have high gamma interferon responses to ESAT6 than do community controls in the Gambia. Infect Immun 2001; 69: 6554-6557.
- Lalvani A, Nagvenkar P, Udwadia Z, Pathan AA, Wilkinson KA, Shastri JS, Ewer K, Hill AV, Mehta A, Rodrigues C. Enumeration of T cells specific RD1- encoded antigens sug-gests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis 2001; 183: 469-477.
- Bhatnagar R. Malviya AN, Narayan S, Rajgopalan P, Kumar R, Bhardwaj OP. Spectrum of immune response abnormalities in different clinical forms of tuberculosis. Am Rev Respir Dis 1977; 115: 207-212.
- David HL, Papa F. Cruaud P, Berlie HC, Maroja MF, Salem JJ, Costa MF. Relationship between titre of antibodies immuno reacting against glycolipid antigens for Mycobacterium leprae and Mycobacterium tuberculosis, the Mitsuda and Mantoux reactions and bacteriologic loads; implications in the pathogenesis, epidemiology and serodiagnosis of leprosy and tuberculosis. Int J lepr other Mycobact Dis 1992; 60: 20824.
- Gerald LB, Bruce F, Brooks CM, Brook N, Kimerling ME, Windsor RA, Bailey WC. Standardizing contact investigation protocols. Int J Tuberc lung Dis 2003; 7: S369-374.