ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2016) Volume 27, Issue 4
Major chromosomal abnormalities and chromosome polymorphism in 1543 couples with recurrent miscarriages in Hubei province of China
Changqing An1#, Dongling Tang2#, Min Wu3#, Xiaofang Ding4# and Xin Jiang5*
1Department of Paediatrics, Hubei Maternal and Child Health Hospital, Wuhan, Hubei, PR China
2Department of Clinical Laboratory, Renmin Hospital of Wuhan University, Wuhan, Hubei, PR China
3Department of Endocrinology, Xiaogan Hospital affiliated to Wuhan Univerity of Sciene and Technology, Xiaogan, HuBei, PR. China
4Center of Reproductive Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, PR China
5Department of orthopaedics, Hubei hospital of traditional Chinese medicine, Wuhan, Hubei, PR China
#These authors contributed equally to this work.
- *Corresponding Author:
- Xin Jiang
Department of Orthopaedics
Hubei hospital of traditional Chinese medicine
PR China
Accepted date: April 26, 2016
Purpose: To examine the major chromosomal abnormalities and chromosome polymorphism in couples with recurrent miscarriagesand provide valuable information for their genetic counselling.
Methods: 1543 couples with three or more times of spontaneous abortions were analysed using Gbanding and fluorescence in situ hybridization (FISH) or array comparative genomic hybridization (aCGH) where ever necessary at the Renmin Hospital of Hubei from January 2012 to December 2014.
Results: Chromosomal anomalies were detected in 314 cases. The abnormalities in number of chromosome were detected in 7 patients. The abnormalities in chromosome structure were detected in 68 cases, including 7 cases for Robertsonian translocation58 cases for reciprocal translocationone case for deletionone case for marker chromosome and one case for chromosomal inversion. Chromosome polymorphisms were detected in 239 patients. This study describes majority of the anomalous cases (except chromosome polymorphism) were balanced reciprocal translocations. Among the abnormal karyotypes we also report two previously undescribed balanced human karyotype.
Conclusions: These findings suggest that chromosomal analysis in couples with recurrent miscarriages should be taken up by all the practioners at all levels. Genetic counsellors should pay attention to the chromosome polymorphism in the couples with recurrent miscarriages.
Keywords
Chromosomal abnormalities, Recurrent miscarriages, Balanced translocations, Chromosome polymorphism.
Introduction
Recurrent miscarriage (RM) is usually defined as the occurrence of 3 or more consecutive pregnancy losses before 20 weeks of gestation or the loss of three consecutive foetuses of less than 500 g in weight [1]. There are different reasons for RM including genetic abnormalities, maternal and paternal age, endocrine dysfunction, autoimmune disorders, infectious diseases, environmental toxins and congenital or structural uterine anomalies [2].
Almost 15–20% of all pregnancies end up as spontaneous miscarriages. It has been reported that 50% of spontaneously aborted foetuses have chromosomal abnormalities [3]. The majority of such abnormalities are due to chromosomal nondisjunction or mutation. Chromosomal abnormalities, mainly balanced rearrangements, are common in couples with reproductive disorders including recurrent abortions. Parental chromosomal abnormalities represent an important etiology of recurrent miscarriages; studies have shown a prevalence of chromosomal anomalies that varies from 2% to 8% of couples who are affected by RM [4]. Unequal crossing over during meiosis can lead to chromosomal rearrangements producing gametes with unbalanced chromosomal aberrations like duplications or deletions, therefore, structural chromosome abnormalities in parents can be the major cause of recurrent miscarriages. The clinical consequences of such imbalances usually are lethal to the developing embryo leading to spontaneous miscarriages or early neonatal deaths [5].
Because the heterochromatic region of chromosome consists of highly repeated sequences of satellite DNA that does not encode proteins, the chromosomal polymorphism variations are considered normal karyotypic variations [6]. However, many recent studies indicate that chromosomal polymorphisms may cause certain clinical effects, such as infertility and recurrent miscarriages [7,8].
The objective of the study was to determine the prevalence and types of chromosomal anomalies and chromosomal polymorphisms in couples with reproductive disorders living in Hubei province of China, to increase the awareness of genetic counsellors about the chromosomal aberrations and chromosomal polymorphisms that contribute to recurrent miscarriages.
Materials and Methods
This study was done from January 2012 to December 2014. A total of 1543 couples with RM were offered chromosomal analysis. In all the cases the detailed reproductive case histories were taken and karyotypes were generated from the peripheral blood lymphocyte cultures and fluorescence in situ hybridization (FISH) or array comparative genomic hybridization (aCGH) were done if necessary at the Renmin Hospital of Hubei.
Metaphase chromosome preparations from the peripheral blood cultures were made according to standard cytogenetic protocols. Cytogenetic analysis was performed by G-banding at approximately 380 band level. C-banding was done to confirm the heterochromatin and satellites on acrocentric chromosomes wherever necessary. FISH (Fluorescence in situ hybridization) was performed to confirm chromosome translocation [9], aCGH (array comparative genomic hybridization) was performed for identifying and analysing chromosome fragment repetition and deletion, for defining the loci of the chromosome fragment repetition [10].
Thirty metaphases were analysed in all the patients but in cases of abnormalities the study was extended to 50 metaphases, in cases of mosaicismhe the study was extended to 100 metaphases. Karyotyping of each couple was carried out according to the International System for Human Cytogenetic Nomenclature (ISCN2013). Along with the structural rearrangements and aneuploidies the chromosome polymorphisms were also studied.
Results
In 1543 couples, we determined that 314 people had chromosomal aberrations (10.17%) (Table 1), among those 68 cases showed structural aberrations (2.07%), 7 numerical anomalies were detected (0.23%) (Table 2), and 239 cases showed normal polymorphic variants (7.74%) (Table 3). Majority of the abnormalities were BRT (Balanced Reciprocal Translocations), (58 cases for reciprocal translocation and 7 cases for Robertsonian translocation).
| Abnormalities | Cases |
|---|---|
| Reciprocal translocation | 58 |
| Robertsonian translocation | 7 |
| Inversion | 1 |
| Deletion | 1 |
| Marker | 1 |
| Aneuploidy | 7 |
| Polymorphic variants | 239 |
| Total | 314 |
Table 1. Total chromosomal abnormality among 1543 couples.
| Abnormalities | Cases |
|---|---|
| Reciprocal translocation | 58 |
| 46, XY, t (4;19) (p14;q12) | |
| 46, XX, t (6;11) (q13;q13) | |
| 46, XX, t (2;15) (q24;q25) | |
| 46, XX, t (10;16) (p13;p11) | |
| 46, XX, t (5;9) (p13;p22) | |
| 46, XX, t (3;6) (q27;p21) | |
| 46, XX, t (6;16) (q23;q22) | |
| 46, XX, t (11;12) (q21;q15) | |
| 46, XX, t (7;14) (p21;q21) | |
| 46, XX, t (10;19) (q22;q12) | |
| 46, XX, t (11;12) (q23;q11) | |
| 46, XX, t (1;14) (q32;q32) | |
| 46, XX, t (8;14) (p11;q32) | |
| 46, XX, t (X;22) (p12;q13) | |
| 46, XX, t (7;10) (p13;q26) | |
| 46, XY, t (4;5) (q33;p14) | |
| 46, XX, t (4;14) (p16;q12) | |
| 46, XY, t (5;16) (q33;p12) | |
| 46, XY, t (9;10) (p13;p13) | |
| 46, XX, t (5;7) (p32;q21) | |
| 46, XX, t (9;22) (q32;q11) | |
| 46, XY, t (10;14) (p13;q24) | |
| 46, XX, t (1;18) (p32;p12) | |
| 46, XY, t (8;14) (p11;q11) | |
| 46, XX, t (4;5) (q23;q34) | |
| 46, XX, t (2;7) (p14;p15) | |
| 46, XX, t (7;16) (q31;q22) | |
| 46, XX, t (4;13) (p11;q11) | |
| 46, XX, t (10;11) (q26;q23) | |
| 46, XY, t (3;14) (p12;q12) | |
| 46, XX, t (4;7) (q33;q21) | |
| 46, XX, t (1;19) (p35;q34) | |
| 46, XX, t (16;20) (p13;p12) | |
| 46, XX, t (3;13) (p21;p11) | |
| 46, XX, t (9;14) (q34;q22) | |
| 46, XY, t (7;14) (q33;q32) | |
| 46, XX, t (7;10) (p23;q21) | |
| 46, XX, t (9;12) (p23;q22) | |
| 46, XY, t (3;9) (q25;q32) | |
| 46, XX, t (7;14) (q35;q23) | |
| 46, XY, t (4;11) (p14;q25) | |
| 46, XY, t (1;9) (q23;q22) | |
| 46, XX, t (1;19) (p13;p12) | |
| 46, XX, t (10;15) (q23;q12) | |
| 46, XX, t (7;9) (p12;p22) | |
| 46, XX, t (1;9) (q11;p12) | |
| 46, XY, t (14;18) (q24;q12) | |
| 46, XY, t (18;22) (q21;q12) | |
| 46, XY, t (6;10) (p24;p11) | |
| 46, XX, t (3;14) (q23;q22) | |
| 46, XX, t (1;13) (q32;q13) | |
| 46, XY, t (1;4) (q33;q13) | |
| 46, XY, t (9;11) (q22;q13) | |
| 46, XX, t (8;10) (q23;q22) | |
| 46, XY, t (6;16) (q25;p12) | |
| 46, X, t (X;13;9) (q21;q13;p22), t (3;6) (p13;q23) | |
| Robertsonian translocation | 7 |
| 45, XX, rob (13;21) | |
| 45, XX, rob (13; 22) | |
| 45, XX, rob (14;15) | |
| 45, XY, rob (13;14) | |
| 45, XY, rob (14;21) | |
| 45, XY, rob (14;15) | |
| 44, XY, rob (14;15) rob (14;15) | |
| Inversion | 1 |
| 46, XX, inv (8) | |
| Marker | 1 |
| 47, XY, +marker | |
| Deletion | 1 |
| 46, XX, del (Xp) | |
| Aneuploidy | 7 |
| 45, X | |
| 47, XXX | |
| 45, X/46, XX | |
| 47, XYY | |
| 47, XXY | |
| 47, XXY | |
| 46, XY/47, XYY | |
Table 2. Structural and numerical chromosomal abnormalities.
| Variants | Cases |
|---|---|
| 46, XN, inv(9) | 37 |
| 46, XN, 1qh+ | 9 |
| 46, XN, 9qh+ | 20 |
| 46, XY, 13ph+ | 2 |
| 46, XY, 15ph+ | 3 |
| 46, XX, 16qh+ | 6 |
| 46, XX, 9qh+, 16ph+ | 1 |
| 46, XX, 13ps+ | 3 |
| 46, XX, 14ps+, 15ps+ | 1 |
| 46, XN, 15ps+ | 5 |
| 46, XN, 21ps+ | 5 |
| 46, XX, 15ps- | 2 |
| 46, XY (long Y) | 63 |
| 46, XY (short Y) | 73 |
| 46, X, inv(Y) | 9 |
| Total | 239 |
Table 3. The identified Polymorphic chromosomal variants.
In this study three novel cases were described.
Case 1
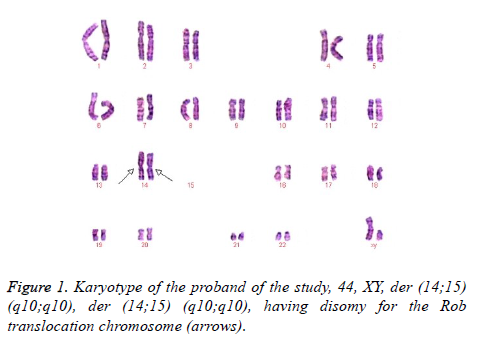
This case was occasioned by the ascertainment of a 25-year-old Chinese man (IV-1) married to a non-consanguineous woman with normal chromosomes (IV-2). This couple had a son who died at the age of 6 months. The karyotyping analysis of IV-1 was authenticated by the Chinese Academic Committee of the state key laboratory of medical genetics with previously undescribed balanced human karyotype 44,XY, der (14;15) (q10;q10), der (14;15) (q10;q10) (Figure 1).
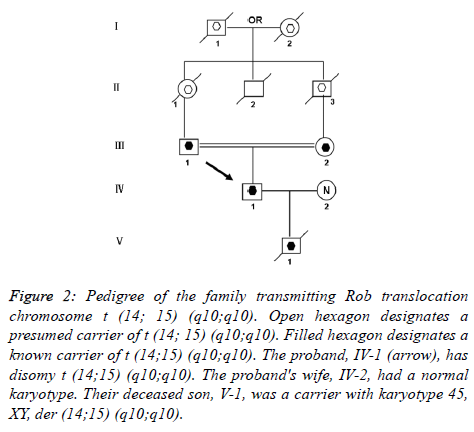
The parents of the propositus are phenotypically normal first consigns, each a carrier of the same Rob translocation (-1, 2). Their parents, a mutual uncle, and both grandparents are deceased, thus it is not possible to determine whether I-1 or I-2 was the carrier of the translocation (Figure 2).
Figure 2. Pedigree of the family transmitting Rob translocation chromosome t (14; 15) (q10;q10). Open hexagon designates a presumed carrier of t (14; 15) (q10;q10). Filled hexagon designates a known carrier of t (14;15) (q10;q10). The proband, IV-1 (arrow), has disomy t (14;15) (q10;q10). The proband's wife, IV-2, had a normal karyotype. Their deceased son, V-1, was a carrier with karyotype 45, XY, der (14;15) (q10;q10).
In order to provide advice for their genetic counselling, we have done sperm FISH for IV-1 and 1.
All semen samples were first analysed to evaluate volume, concentration and motility (Table 4), according to the World Health Organization criteria [11]. After semen analysis, two sets of probe mixtures were used in this study: Firstly, for the detection of normal/balanced or unbalanced sperm, dual-colour FISH was carried out using locus specific probes (LSP) and Tel probes from Vysis (Vysis, Downers Grove, IL, USA). For IV-1 and 114/15 two fluorescent probes were used: TelVysion Probe 14q (D14S1420, Spectrum Red) for 14q32.33 and TelVysion Probe 15q (D15S120, Spectrum Green) for 15q26.3. Secondly, to investigate the presence of ICE, triple-color FISH was performed using the second probe mixture which consist of commercial satellite (DNA) probes from Vysis, including chromosomes 18, X and Y (CEP 18, Spectrum Blue/CEP X, Spectrum Green/CEP Y, Spectrum Red) [12].
| Patient | Age (years) | Sperm concentration (×106/ml) | Mobility (a+b)\ (%) |
|---|---|---|---|
| III-1 | 48 | 13 | 22 |
| IV-1 | 25 | 56 | 53 |
Table 4. Cytogenetic and spermiologic results of IV-1 and 1.
A total of 4,000 sperm nuclei from the two translocation carriers were analysed for this study. The results of the segregation analysis are detailed in Table 5 and Table 6.
| Segregation modes | III-1 | IV-1 |
| Normal or balanced | 799 | 997 |
| Nullisomy 14 | 41 | 2 |
| Disomy 14 | 49 | 0 |
| Nullisomy 15 | 55 | 0 |
| Disomy 15 | 45 | 0 |
| 3:0 or diploid | 11 | 1 |
| Total | 1000 | 1000 |
Table 5. The number of spermatozoa scored the alternate mode of segregation, incidence of sperm nullisomy, disomy and 3:0 /diploid for the chromosomes involved in the Rob translocation in two Rob translocation carriers.
| Segregation modes | III-1 | IV-1 |
| Normal or balanced | 949 | 994 |
| Nullisomy 18 | 3 | 0 |
| Disomy 18 | 2 | 1 |
| Nullisomy Sex chromosome | 15 | 2 |
| Disomy Sex chromosome | 10 | 3 |
| 3:0 or diploid | 21 | 0 |
| Total | 1000 | 1000 |
Table 6. Incidence of sperm nullisomy, disomy and diploid for chromosomes 18, X and Y in two Rob translocation carriers.
To Rob translocation heterozygote of this paper (1), the rate of normal/balanced spermatozoa resulting from alternate segregation is 79.9%. The frequency of unbalanced spermatozoa resulting from adjacent segregation is 20.1%. To Rob translocation homozygosity (IV-1), the rate of balanced spermatozoa is 99.7%. The frequency of unbalanced spermatozoa is 0.3% (Table 2). In 1, the frequency of unbalanced spermatozoa was significantly higher compared to that of IV-1 (P<0.05). In IV-1, the frequency of unbalanced spermatozoa was similarity to some controls [13-17].
The nullisomy, disomy and diploid rates for chromosomes 18, X and Y in 1 and IV-1 are summarized in Table 3. In 1, the higher frequencies of aneuploidy for sex chromosome were observed. The incidence of spermatozoa with nullisomy disomy and diploid for the sex chromosomes of 1 was significantly higher compared to that of IV-1 (P<0.05). But, compared IV-1 with controls, the incidence was not significant [13-17].
Because the rate of balanced sperm of IV-1 is 99.7%, we suggested that they can try to conceive naturally, and now they have a normal phenotype son whose karyotype is 45, XY, der (14;15) (q10;q10).
Case 2
A 29-year old woman with normal phenotype was referred for chromosomal analysis due to RM. Cytogenetic analysis had revealed a previously undescribed karyotype of 46, X, t(X; 13;9) (q21;q13;p22), t(3;6) (p13;q23) (Figure 3). Because of her karyotype, we suggested that she could choose PGD (preimplantation genetic diagnosis) or to adopt child.
Case 3
A 35 year-old man was referred for chromosomal analysis due to RM of his identical twin. His karyotype showed 47, XY, +mar.
Array comparative genomic hybridization (aCGH) has been introduced in clinical diagnosis to rapidly detect genome wide gains and losses with higher resolution [18].
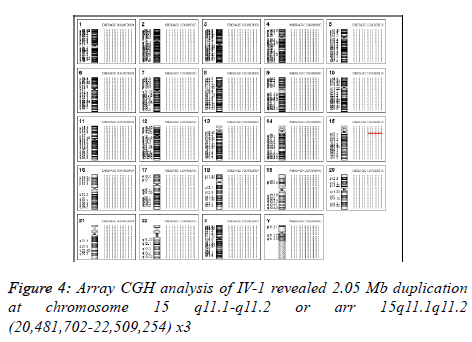
We have done aCGH for him in order to find out the source of the small supernumerary marker chromosome and aCGH using Agilent’s 8 × 60 K commercial arrays (Agilent Technologies, Santa Clara, CA, USA) was performed on DNA extracted from peripheral blood [19]. 2.05 Mb duplication was detected at chromosome 15 q11.1-q11.2 or arr 15q11.1q11.2 (20,481,702-22,509,254) x3 (Figure 4). By checking the CNV database, we know that this duplication is a kind of chromosomal polymorphism in normal population.
Because of these, we suggested that he can try to conceive naturally, and now he has a daughter whose karyotype is 46, XX.
Discussion
The most likely pathogenic mechanism behind RM is a multifactorial mode of inheritance. Several causes such as single gene mutations, genomic imprinting, chromosomal instability, and sperm chromosome abnormalities have been suggested to explain idiopathic reproductive loses. Cytogenetic studies give considerable information about the genetic makeup leading to RM and still remain an important tool. To the couples with RM, the risk of finding a chromosomal anomaly (especially structural chromosomal anomaly) is significantly higher [2]. Because of such rearrangements, the chromosomes have difficulty in pairing up and dividing evenly during meiosis. So the carriers of BRT have a risk of partial trisomy or partial monosomy for chromosomal regions involved in the translocation.
In this study, chromosomal anomalies were detected in 314 cases are done by using G- banding FISH and aCGH. The abnormalities in number of chromosome were detected in 7 patients. The abnormalities in chromosome structure were detected in 68 cases, including 7 cases for Robertsonian translocation58 cases for reciprocal translocationone case for deletionone case for marker chromosome and one case for chromosomal inversion. Chromosome polymorphisms were detected in 239 patients.
This study describes majority of the anomalous cases were balanced reciprocal translocations, 58/75, 77.3% detected in the current study as has also been reported in other studies [4]. There were more subsequent miscarriages among carriers of translocation. For carriers of translocation, there were poorer prognosis in carriers of translocation, with a higher rate of subsequent miscarriages and lower rate of viable pregnancies.
Numerical chromosomal aberrations are less frequent among abnormal couples with recurrent abortions (7/75, 9.3%). These types of aberrations are usually in the form of sex chromosomal aneuploidy. The current study showed that the incidence and distribution of chromosomal abnormalities with recurrent abortions are comparable to that reported worldwide. The prevalence and type of chromosomal abnormalities is similar to that seen in other reports [20-22].
The role of polymorphic variants of chromosomes in RM has not yet verified. In recent years, more and more studies have shown an increased incidence of chromosomal polymorphism variation in RM couples [23].
We found chromosomal polymorphism just as inv (9), 1 qh+, 9 qh+, 16 qh+, 13 ph+, 15 ph+, 15 ps+, 21 ps+, 15 ps-, long Y, short Y and inv (Y) in this study.
The most frequent chromosomal polymorphism in the general human population is inv (9). This inversion is usually a normal polymorphism; however, its clinical consequences remain unclear [24]. In addition, the additional G-band in the cases of 9 qh+ and 16 qh+ is not believed to be an etiological cause of abortion because this band is in the heterochromatin blocks, which contain no active genes [25,26].
Conclusions
Present study confirmed that chromosomal abnormalities are common in couples having recurrent miscarriages. We discussed the significance of balance translocation and chromosomal polymorphism in RM couples.
The findings in our sample are consistent with figures described in several populations around the world. The overall incidence of chromosomal abnormalities indicates that chromosomal analysis of the couples with RM should be essentially considered. Among the novel karyotypes, we report for the first time two unique cases of chromosomal translocation associated with RM.
Genetic counsellors should pay attention to the chromosomal anomalies and polymorphism in the couples with recurrent miscarriages.
Acknowledgement
Supported by a grant 81102141 from National Science Foundat ion of China and another grant WJ2015Q015 from youth Science Foundatio n of Hubei Province
References
- Rai R, Regan L. Recurrent miscarriage. Lancet. 2006; 368:601-11.
- Goncalves RO, Santos WV, Sarno M, Cerqueira BA, Goncalves MS, Costa OL. Chromosomal abnormalities in couples with recurrent first trimester abortions. Rev Bras Ginecol Obstet. 2014; 36:113-7.
- Ghazaey S, Keify F, Mirzaei F, Maleki M, Tootian S, Ahadian M, Abbaszadegan MR. Chromosomal analysis of couples with repeated spontaneous abortions in north eastern Iran. Int J Fertil Steril. 2015; 9: 47-54.
- Elghezal H, Hidar S, Mougou S, Khairi H, Saad A. Prevalence of chromosomal abnormalities in couples with recurrent miscarriage. Fertil Steril. 2007; 88: 721–723.
- Rao L, Murthy K, Babu A, Venkata P, Deenadayal M, Singh L. Chromosome inversions and a novel chromosome insertion associated with recurrent miscarriages in South India. Arch Gynecol Obstet. 2005; 272: 273–277.
- Yuce H, Tekedereli I, Elyas H. Cytogenetic results of recurrent spontaneous miscarriages in Turkey. Med Sci Monit. 2007; 13: CR 286-CR 289.
- Bhasin MK. Human population cytogenetics: a review. Int J Hum Genet. 2005; 5: 83-152.
- Sahin FI, Yilmaz Z, Yuregir OO, Bulakbasi T, Ozer O, Zeyneloglu HB. Chromosome heteromorphisms: an impact on infertility. J Assist Reprod
- Genet. 2008; 25: 191-195.
- Shuqin XU, Dongling T, Kun F, BO W. Analysis of meiotic segregation patterns and interchromosomal effects in sperm from a Robertsonian translocation family. Biomed Res 2014; 25: 233-239.
- ACOG Committee Opinion No. 446: array comparative genomic hybridization in prenatal diagnosis. Obstet Gynecol 2009, 114:1161–1163.
- World Health Organisation. WHO laboratory manual for the examination and processing of human semen. 5 ed. Geneva, Switzerland; 2010.
- Anton E, Vidal F, Blanco J. Role of sperm FISH studies in the genetic reproductive advice of structural reorganization carriers. Hum Reprod 2007; 22: 2088-2092.
- Machev N, Gosset P, Warter S, Treger M, Schillinger M, Viville S. Fluorescence in situ hybridization sperm analysis of six translocation carriers provides evidence of an interchromosomal effect. Fertility and Sterility 2005; 84: 365-373.
- Mahjoub M, Mehdi M, Brahem S, Elghezal H, Ibala S, Saad A. Chromosomal segregation in spermatozoa of five Robertsonian translocation carriers t(13;14). J Assist Reprod Genet. 2011; 28: 607-613.
- Rouen A, Pyram K, Pollet-Villard X, Hyon C, Dorna M, Marques S. Simultaneous cell by cell study of both DNA fragmentation and chromosomal segregation in spermatozoa from chromosomal rearrangement carriers. J Assist Reprod Genet. 2013; 30: 383-390.
- Cassuto NG, Foll NL, Chantot-Bastaraud S, Balet R, Bouret D, Rouen A. Sperm fluorescence in situ hybridization study in nine men carrying a Robertsonian or a reciprocal translocation: relationship between segregation modes and high-magnification sperm morphology examination. Fertil Steril. 2011; 96: 826-832.
- Ferfouri F, Selva J, Boitrelle F, Gomes DM, Torre A, AlbertM. The chromosomal risk in sperm from heterozygous Robertsonian translocation carriers is related to the sperm count and the translocation type. Fertil Steril. 2011; 96: 1337-1343.
- ACOG Committee Opinion No. 446: array comparative genomic hybridization in prenatal diagnosis. Obstet Gynecol 2009; 114:1161–1163.
- Liu Y, Nakatsukasa K, Kotera M. Non-SCF-type F-box protein Roy1/Ymr258c interacts with a Rab5-like GTPase Ypt52 and inhibits Ypt52 function. Mol Biol Cell. 2011; 22:1575-1584.
- Niroumanesh S, Mehdipour P, Farajpour A, Darvish S. A cytogenetic study of couples with repeated spontaneous abortions. Ann Saudi Med. 2011; 31: 77-79.
- Pal S, Ma SO, Norhasimah M, Suhaida MA, Siti Mariam I, Ankathil R, et al. Chromosomal abnormalities and reproductive outcome in Malaysian couples with miscarriages. Singapore Med J. 2009; 50: 1008-1012.
- Goud TM, Mohammed Al Harassi S, Khalfan Al Salmani K, Mohammed AL Busaidy S, Rajab A. Cytogenetic studies in couples with recurrent miscarriage in the Sultanate of Oman. Reprod Biomed Online. 2009; 18: 424-429.
- Caglayan AO, Ozyazgan I, Demiryilmaz F, Ozgun MT. Are heterochromatin polymorphisms associated with recurrent miscarriage? J Obstet Gynaecol Res. 2010; 36: 774-776.
- Dana M, Stoian V. Association of pericentric inversion of chromosome 9 and infertility in romanian population. Maedica (Buchar). 2012; 7: 25-29.
- Celep F, Karagüzel A, Ozeren M, Bozkaya H. The frequency of chromosomal abnormalities in patients with reproductive failure. Eur J Obstet Gynecol Reprod Biol. 2006;127: 106-109.