ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2018) Artificial Intelligent Techniques for Bio Medical Signal Processing: Edition-II
Metformin prevents proliferation of prostate cancer by regulating IGF1R/PI3K/Akt signalling in a mouse model
Dongfang Jiang*, Qiang Bu, Minghui Zeng, Suobing Qin, Dongdong Xia and Yi Peng
Department of Urology, Danyang People's Hospital, No. 2, Xinmin West Road, Danyang City, Jiangsu Province, PR China
Accepted on December 04, 2017
DOI: 10.4066/biomedicalresearch.29-17-3103
Visit for more related articles at Biomedical ResearchObjective: To elucidate the inhibition mechanism of metformin on prostate cancer growth, we studied the effect of metformin in a mouse model.
Methods: Six-week old male BALB/c nude mice in SPF level were divided into three groups after tumour initiation and were administered once daily intraperitoneally for 3 weeks: control group, metformin-treated group and picropodophyllin-treated (PPP, IGF1R inhibitor) group. We assessed the effect of metformin on tumour volume, tumour weight and IGF1R/PI3K/Akt signalling pathway in mice.
Results: Metformin treatment significantly reduced tumour weight compared with those of untreated control group and picropodophyllin-treated group. IGF-1R mRNA levels and PI3K, p-Akt and Akt protein levels of metformin-treated group were significantly decreased than control group and picropodophyllin-treated group.
Conclusion: Metformin inhibits the growth of prostate cancer by inhibiting IGF1R/PI3K/Akt signalling axis and it may be beneficial for prostate cancer treatment.
Keywords
Metformin, IGF1R, Prostate cancer, Picropodophyllin
Introduction
Prostate cancer is the second common male malignant tumours worldwide. It is reported there are 6-7 million new cases every year in China, becoming a threat to the health of elderly men [1]. About 30% patients have been developed into advanced stage of prostate cancer when diagnosed, and there is less chance of surgical resection [2]. After the endocrine therapy for 18-24 months, large of patients would step into castrationresistant prostate cancer (CRPC) with a poor clinical prognosis [3]. Earlier studies confirmed hyperinsulinemia and insulin resistance were independent risk factors of prostate cancer [4]. Metformin, as an insulin sensitizer, reduces hyperinsulinemia and possesses anti-cancer effects to colon cancer, ovarian cancer, prostate cancer [5-7]. In addition, piles of studies showed insulin-like growth factor 1 receptor (IGF1R) played a crucial role in tumour cell growth, apoptosis, development, other than glucose and lipid metabolism [8]. It was demonstrated that targeting IGF-1R/Akt signalling pathway with glucosamine was effective for treating human non-small cell lung cancer [9].
In this study, we implanted prostate tumour cell line into prostate of immunocompetent rat and investigated the response of prostate tumours to metformin. Further, we evaluated the inhibition mechanism of metformin on prostate cancer via mediating IGF1R/PI3K/Akt signal axis in prostate tumour tissues, to provide new targets and theoretical basis for tumorigenesis and intervention.
Materials and Methods
Cells and animals
DU145 human prostate tumour cell lines were purchased from Cell Bank of Shanghai Institutes for biological Sciences (Chinese Academy of Sciences). Cells were cultured in RPMI 1640 (Gibco) medium containing 10% foetal bovine serum (FBS) at 37°C with 5% CO2. Cells were harvest by 0.5% trypsin for 3 min and washed by 4°C ice-cold sterile PBS.
A total of 21 BALB/c male nude mice aged 4-6 weeks in SPF level with an average weight of 18-20 g were purchased from Shanghai Sangon Cell Experimental Center, and were housed in a well-ventilated room under 25°C with free access of food and water. 2 × 104 cells were suspended in 10 μL PBS and were carefully injected into one of the ventral prostate lobes continuously for 7d. Tumour-bearing mice were divided into three groups, including control group (n=7), metformin-treated group (n=7) and picropodophyllin-treated group (n=7). Control group received 150 μL saline injection i.p. Metformin-treated group received metformin (120 mg/kg in 150 μL saline). Picropodophyllin-treated group was dosing with picropodophyllin (0.1 μM in 150 μL saline) 4h before metformin (120 mg/kg in 150 μL saline) treatment.
Mice were sacrificed 10 days after 3-week agent treatment. The tumour-containing prostates were removed, weighed, frozen in liquid nitrogen, and stored in -80°C. Tissues from untreated mice were served as controls. Metformin and picropodophyllin was bought from Sigma-Aldrich.
Quantification of mRNA levels by real-time qPCR
Total RNA in tumour tissues were extracted by Allprep RNA mini kit (Qiagen). The purity of RNA samples was evaluated by NanoDrop™ One (Thermo fisher). Primers were synthesized by Shanghai Sangon Company. IGF1R: F: 5’- ATGCTGTTTGAACTGATGCGCA-3’, R: 5’- GCCCCCGGCGTTCTTGCTCGCC-3’, 354bp; GAPDH, F: 5’- CGCGAGAAGATGACCCAGAT-3’, R: 5’- GCACTGTGTTGGCGTACAGG-3’, 169 bp. The cDNA was synthesized from 0.5 μg of RNA with a Prime Script Kit (TAKARA, Osaka, Japan). Relative mRNA levels of IGF-1R were quantified by real-time PCR (RT-qPCR) using a Bio-Rad C1000 qPCR Detection System and Power SYBR Green PCR Master Mix as recommended by the manufacturer (Life Technologies). The relative mRNA levels were presented via 2- Ct method.
Protein extraction and western-blot
After tissue homogenate, total cell protein was extracted in a buffer consisted of 25 mM HEPES buffer, pH 7.9, 1 U/μLof Benzonase 1 mM MgCl, 5 mM EDTA and PhosSTOP (Roche) per 10 ml of buffer. 30μg protein was mixed with 4 × Laemmli buffer Laemmli buffer (Bio-rad, Hercules, CA). After boiled, proteins from each sample were separated by SDS-PAGE and transferred to PVDF membrane (Millipore, Billerica, MA, USA). After blocked by non-fat milk for 1h, the membranes were incubated with primary antibodies (PI3K p110α, 1:1000; phospho-AKT (Ser473), 1:1000; AKT,1:1000; β-actin; 1:5000) and Horseradish peroxidase (HRP)-conjuncted secondary antibody (1:1000). All antibodies were obtained from abcam. Reactive bands were by using a chemiluminescence (ECL) detection kit (Millipore).
Statistical analyses
SPSS 13.0 software was used for statistical analysis. Measurement data was presented as mean ± standard deviation. The volumes (V) of the excised tumours were measured with an external calliper and calculated as V=0.52 (length × width × depth). One-way ANOVA was used for comparison among groups. LSD-t test was used for post-hoc comparison. P<0.05 was considered as statistically significant.
Results
Metformin inhibits prostate tumour cell growth
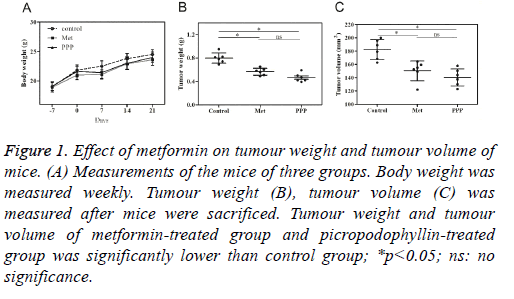
During the period of the experiment, no significant difference in body weight was found in 3 groups (Table 1, Figure 1A). There was no death of mice before sacrificed. The tumour weight was 0.79 ± 0.19 g, 0.6 ± 0.12 g and 0.47 ± 0.15 g for control group, metformin-treated group and picropodophyllintreated group. The average tumour volume was (195.6 ± 32.4) mm3 in control group, (157.8 ± 21.3) mm3 in metformintreated group, and (164.3 ± 24.2) mm3 in picropodophyllintreated group. Tumour weight in metformin-treated group and picropodophyllin-treated group decreased when compared with control group, with the differences statistically significant (Figure 1B, p<0.05), whereas no significant difference was found between metformin-treated group and picropodophyllintreated group. Similar results were found in tumour volume (Figure 1C, p<0.05).
| Variable | Control (n=7) | Met (n-7) | PPP (n=7) |
|---|---|---|---|
| Pancreatic tumor weight, g | 0.79 ± 0.19 | 0.6 ± 0.12 | 0.47 ± 0.15 |
| Pancreatic tumor volume, mm3 | 195.6 ± 32.4 | 157.8 ± 21.3 | 164.3 ± 24.2 |
Table 1. Characteristics of three groups of mice.
Figure 1: Effect of metformin on tumour weight and tumour volume of mice. (A) Measurements of the mice of three groups. Body weight was measured weekly. Tumour weight (B), tumour volume (C) was measured after mice were sacrificed. Tumour weight and tumour volume of metformin-treated group and picropodophyllin-treated group was significantly lower than control group; *p<0.05; ns: no significance.
Metformin inhibits the expression of IGF-1R mRNA
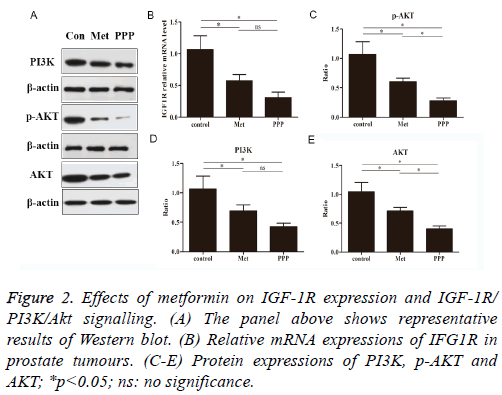
The effects of metformin were investigated by real-time qPCR. We determined whether metformin blocked the IGF1R in prostate tumour of mice. As shown in Figure 2B, metformin treatment as well as picropodophyllin treatment significantly reduced IGF1R mRNA expressions compared to control group. No significance was observed between metformin-treated group and picropodophyllin-treated group.
Metformin alleviates PI3K, Akt and phosphorylated Akt (p-Akt) protein levels
We examined the effect of metformin on PI3K, p-Akt and Akt activation by looking for changes in the level of total protein (Figure 2A). Metformin and picropodophyllin treatment significantly attenuated the activation of PI3K and AKT and phosphorylation of AKT on Ser473, compared to control group (Figures 2C-2E, p<0.05). Picropodophyllin treatment significantly suppressed the activation of AKT and phosphorylation of AKT on Ser473 (Figures 2C-2E, p<0.05).
Discussion
It is known that metformin inhibits tumour through activating AMP-activated protein kinase (AMPK), thereby inhibiting mTOR pathway activity. Moreover, metformin could downregulate Cyclin D1 and inhibit tumour cell mitosis [10]. Data has shown that [11] metformin can reduce hepatic gluconeogenesis, stimulate muscle tissues to take in glucose and reduce insulin levels and reduce hyperinsulinemia through mediating AMPK. Under high insulin concentration, metformin was able to activate the downstream ERK and PI3K signalling pathways through binding to insulin receptor (IR) and IGF1R on tumour cell membrane, consequently promoted the malignant progression of tumour cells. However, the mechanism of metformin of inhibiting tumorigenesis and development may not involve the changes in blood glucose and insulin levels, but the influence on IGF1R/STAT3 signal axis [12].
As mentioned, metformin can inhibit the development of castration-resistant prostate cancer and reduce the mortality of prostate cancer. It has been showed that metformin treatment for 12226 patients with prostate cancer significantly reduce the risk of prostate cancer [13,14]. Clinical data from more than 350 primary care centres in Britain for retrospective cohort study and found that metformin treatment can improve the survival rate of malignant tumour patients with type 2 diabetes mellitus [15]. In this study, we did observe a significant decrease of tumour weight and tumour volume induced by metformin, suggesting that metformin had potential to be a novel approach for prostate tumour prevention.
Accordingly, when IGF1 or insulin binds to the receptors IGF1R thereby inducing a change in the transmembrane β subunits, phosphorylation of IRS is able to activate phosphatidylinositol 3-kinase (PI3K) and consequently of AKT. Picropodophyllin has been reported to specifically inhibit IGF1R activity via blocking IGF1R phosphorylation and downstream signalling, including Akt and MAPK phosphorylation [16,17]. Also, picropodophyllin induces apoptosis of malignant cells in different tumour models [18,19]. At present, exact mechanism of action of metformin on prostate cancer has not been fully elucidated. It remains unknown whether metformin inhibits prostate cancer progression through IGF1R /PI3K/Akt signal axis. In this study, we showed that metformin effectively inhibits IGF-1Rmediated PI3K/Akt signal transduction in prostate tumour.
Taking metformin helps prevent prostate cancer and improve the prognosis of high-grade prostate cancer, which is a conventional drug in new use.
Acknowledgement
The study was supported by Main Special Projects of Health Science and Technology, Zhenjiang City, China (No.: FZ2015085).
References
- Siegel R, Naishadham D, Jemal A.v Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11-30.
- Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513-1520.
- Cox H, Hames M, Benrashid M. Radium-223 for the Management of Bone Metastases in Castration-Resistant Prostate Cancer. J Adv Pract Oncol 2015; 6: 565-570.
- Nandeesha H, Koner BC, Dorairajan LN, Sen SK. Hyperinsulinemia and dyslipidemia in non-diabetic benign prostatic hyperplasia. Clin Chim Acta 2006; 370: 89-93.
- Thompson MD, Lubet RA, Mccormick DL, Clapper ML, Bode AM, Juliana MM, Moeinpour F, Grubbs CJ. Lack of chemopreventive efficacy of metformin in rodent models of urinary bladder, head and neck, and colon/intestine cancer. Oncol Lett 2017; 14: 3480-3486.
- Mert I, Chhina J, Allo G, Dai J, Seward S, Carey MS, Llaurado M, Giri S, Rattan R, Munkarah AR. Synergistic effect of MEK inhibitor and metformin combination in low grade serous ovarian cancer. Gynecol Oncol 2017; 146: 319-326.
- Kato H, Sekine Y, Furuya Y, Miyazawa Y, Koike H, Suzuki K. Metformin inhibits the proliferation of human prostate cancer PC-3 cells via the downregulation of insulin-like growth factor 1 receptor. Biochem Biophys Res Commun 2015; 461: 115-121.
- Heidegger I, Massoner P, Sampson N, Klocker H. The insulin-like growth factor (IGF) axis as an anticancer target in prostate cancer. Cancer Lett 2015; 367: 113-121.
- Song KH, Kang JH, Woo JK, Nam JS, Min HY, Lee HY, Kim SY, Oh SH. The novel IGF-IR/Akt-dependent anticancer activities of glucosamine. BMC Cancer 2014; 14: 31.
- Schneider MB, Matsuzaki H, Haorah J, Ulrich A, Standop J, Ding XZ, Adrian TE, Pour PM. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology 2001; 120: 1263-1270.
- Becker S, Dossus L, Kaaks R. Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Arch Physiol Biochem 2009; 115: 86-96.
- Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le Marchand-Brustel Y, Bost F. The antidiabetic drug metformin exerts an antitumoral effect invitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008; 27: 3576-3586.
- Preston MA, Riis AH, Ehrenstein V, Breau RH, Batista JL, Olumi AF, Mucci LA, Adami HO, Sørensen HT. Metformin use and prostate cancer risk. Eur Urol 2014; 66: 1012-1020.
- Tseng CH. Metformin significantly reduces incident prostate cancer risk in Taiwanese men with type 2 diabetes mellitus. Eur J Cancer 2014; 50: 2831-2837.
- Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012; 35: 299-304.
- Vasilcanu D, Girnita A, Girnita L, Vasilcanu R, Axelson M, Larsson O. The cyclolignan PPP induces activation loop-specific inhibition of tyrosine phosphorylation of the insulin-like growth factor-1 receptor. Link to the phosphatidyl inositol-3 kinase/Akt apoptotic pathway. Oncogene 2004; 23: 7854-7862.
- Menu E, Jernberg-Wiklund H, Stromberg T, De Raeve H, Girnita L, Larsson O, Axelson M, Asosingh K, Nilsson K, Van Camp B, Vanderkerken K. Inhibiting the IGF-1 receptor tyrosine kinase with the cyclolignan PPP: an in vitro and in vivo study in the 5T33MM mouse model. Blood 2006; 107: 655-660.
- Girnita A, Girnita L, del Prete F, Bartolazzi A, Larsson O, Axelson M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res 2004; 64: 236-242.
- Yin SC, Guo W, Tao ZZ. Picropodophyllin inhibits tumor growth of human nasopharyngeal carcinoma in a mouse model. Biochem Biophys Res Commun 2013; 439: 1-5.