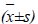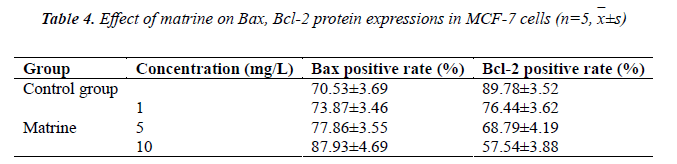ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 3
Method for quantitative determination of matrine in Sophora alopecuroides L. and its inhibitory effect on breast cancer MCF-7 cell proliferation.
Yongyun Shi1,2, Guodong Shen3, Hanlin Fang4, Chuankui Xu2, Shilian Hu5*
1Department of Obstetrics and Gynecology, Hefei Hospital affiliated to Anhui Medical University, Hefei, 230001, China;
2Department of Obstetrics and Gynecology, Hefei second people's Hospital, Hefei, 230001, China
3Anhui Province Key Laboratory of Molecular Medicine, Hefei, 230001, China
4Department of Thoracic Surgery, Hefei second people's Hospital, Hefei, 230001, China
5Department of Geriatrics, Anhui Provincial Hospital affiliated to Anhui Medical University, Hefei?230001, China
- *Corresponding Author:
- Shilian Hu
Department of Geriatrics
Anhui Provincial Hospital
affiliated to Anhui Medical University
17 Lujiang Road, Hefei 230001 China
Accepted May 28 2014
To explore the method for quantitative determination of active constituent in Sophora alopecuroides L. and its anti-cancer activity. Method for quantitative determination of matrine in Sophora alopecuroides L. is established using HPLC with CLC-phenyl column, mobile phase of acetonitrile-anhydrous ethanol-3% phosphoric acid solution (80:10:10), detection wavelength of 220 nm and flow rate of 1.0 mL·min-1. Breast cancer MCF-7 cells are cultured by routine method. Inhibitory effect of matrine on breast cancer MCF-7 cell proliferation is determined by MTT assay. Flow cytometry is used to analyze the changes in cell cycle after treatment, and record percentages of Bax and Bcl-2 positive cells. 48 h after treatment with test concentrations of matrine, cell cycle of MCF-7 cells are evidently altered. With the addition of matrine, S phase MCF-7 cells are markedly reduced, and G0/G1 phase cells markedly increase, while G2/M phase cells do not change much. Flow cytometry results show that the test concentrations of matrine can effectively inhibit the viability of MCF-7 cells, and promote their apoptosis. Different concentrations of matrine can all somewhat increase the positive rate of Bax expression, and the effect exhibits an increasing trend with increasing concentration. Bcl-2 expressions of treatment groups are all evidently lower than the control group, showing a negative correlation. HPLC method is reliable and accurate in determining alkaloids in Sophora alopecuroides L., and matrine in Sophora alopecuroides L. can effectively inhibit the proliferation of breast cancer MCF-7 cells.
Keywords
Sophora alopecuroides L.; matrine; breast cancer MCF-7 cell
Introducation
Kudouzi, also known as Kudougen or Kugancao, is the dried whole plant, root and seed of Sophora alopecuroides L. in the genus Sophora of the family Leguminosae, which is distributed mainly in China's western provinces such as Xinjiang, Inner Mongolia, Ningxia and Gansu [1]. Sophora alopecuroides L. is included in the 2010 Edition of the Chinese Pharmacopoeia. It is commonly used for antisepsis, anti-inflammation, insecticide and antidysentery in clinical settings, and is used in the treatment of dysentery, eczema, stubborn tinea, trichomoniasis, stomachache, etc [2]. Main chemical constituents of Sophora alopecuroides L. are alkaloids and flavonoids; besides, it also contains volatile oils, organic acids, polysaccharides, coumarins, inorganic elements, fatty acids, free amino acids, etc [3-7].
Studies in recent years have demonstrated that Sophora alopecuroides L. has a variety of pharmacological actions such as sedative, analgesic, anticonvulsant, antiarrhythmic, anti-inflammatory, hypotensive and immunomodulatory effects [8-10]. In addition, Jiao Heling et al.'s[11] study has shown that total alkaloids in Sophora alopecuroides L. have a marked inhibitory effect on proliferation of in-vitro cultured SW480 cells, and can induce their apoptosis; furthermore, the effect is dose- and timedependent. Other studies have shown that Sophora alopecuroides L. can inhibit the proliferation of colon, liver and tongue cancer cells, which possesses rather evident anti-tumor activity [12-15]. Thus it can be seen that Sophora alopecuroides L. has broad development prospects.
This study establishes the RP-HPLC conditions to directly isolate and determine matrine in Sophora alopecuroides L. on the basis of previous work. The method is simple, efficient, exclusive and accurate. Meanwhile, inhibitory effect of matrine on human breast cancer cell proliferation is explored, thereby providing the basis for quantitative analysis and expanded utilization of Sophora alopecuroides L.
Materials
Instruments and reagents
Shimadzu LC-10A HPLC system, SPD-10A UV-Vis detector. Acetonitrile and phosphoric acid were of HPLC grade, and the remaining reagents were all of analytical grade. MTT, DMSO, and PI were purchased from Sigma; SW-CJ- 2F ultra clean bench was purchased from Suzhou Purification Equipment Factory; FBS was purchased from Gibco; and RPMI 1640 medium was purchased from Invitroogen.
Drugs and cells
Sophora alopecuroides L. was collected from Ningxia region, which was identified as Sophora alopecuroides L. in the genus Sophora of the family Leguminosae. Matrine reference substance was purchased from the National Institutes for Food and Drug Control, matrine was extracted from Sophora alopecuroides L., and MCF-7 cells were purchased from Shanghai X-Y Biotechnology Co., Ltd.
Methods
Chromatographic conditions
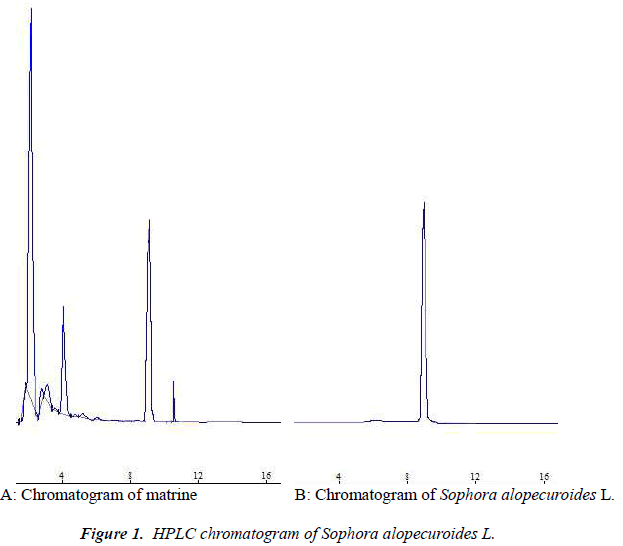
Column: CLC-phenyl column (Shimadzu, Japan); mobile phase: acetonitrile-anhydrous ethanol-3% phosphoric acid solution (80:10:10); detection wavelength: 220 nm; flow rate: 1.0 mL·min-1; detection was performed by external standard method at room temperature. Number of theoretical plates was calculated to be not less than 10,000 based on the matrine peak, and resolutions between matrine and adjacent peaks were all greater than 1.5. See Figure 1.
Linearity range
Reference solution
Appropriate amount of matrine reference substance was accurately weighed, and dissolved in mobile phase to prepare the 0.05mg·mL-1 reference solution.
Test solution was prepared as per the method under Sophora alopecuroides L. section in the Chinese Pharmacopoeia. About 0.5 g of Sophora alopecuroides L. was accurately weighed, placed in a stoppered Erlenmeyer flask, added with 0.5 mL of concentrated ammonia solution and 20 mL of chloroform, stoppered, weighed, ultra sonicated (power: 250 w, frequency: 33 kHz) for 30 min, then let cool, and weighed again. After the lost weight was replenished with chloroform, the solution was shaken well, and filtered. 5 mL of subsequent filtrate was precisely taken, passed through a neutral alumina column (100-200 mesh, 5 g, inner diameter: 1 cm), and eluted sequentially with each 20 mL of chloroform and chloroform- methanol (7:3) mixed solution. Next, all eluents were collected, and solvents were removed to dryness. Finally, the residue was dissolved in mobile phase, transferred to a 10 mL volumetric flask, diluted to the mark with anhydrous ethanol, and shaken well to give the test solution.
Linearity range
The above reference solution was prepared into 0.04, 0.06, 0.08, 0.10, 0.12 and 0.14 mg·mL-1 solutions, and determined according to the chromatographic conditions under section 2.1. Peak area was used as ordinate, and mass concentration of matrine was used as abscissa. After linear regression, regression equation was obtained as: Y = 283412.8X + 67632.r = 0.999 5, indicating that matrine had a good linearity within a 0.04-0.14 mg·mL-1 range.
Stability test
Test solution was determined according to the method 0, 1, 2, 4, 6 and 12 h after preparation, respectively. The results showed that the test solution was basically stable within 0-12 h. No significant changes were observed in the determination results, and RSD of matrine peak area was 0.53%, indicating good stability within 12 h.
Accuracy test
Each 5 uL of 0.05 mg·mL-1 matrine reference solution was injected 6 repeated times, and RSD of matrine peak area was 0.18%.
Recovery test
Appropriate amount of matrine reference substance was added with anhydrous ethanol to prepare a 0.05mg·mL-1 solution. Six aliquots of 0.5 g of Sophora alopecuroides L. medicinal powders were accurately weighed, added precisely with 10 mL of the above reference solution, respectively, to prepare the desired solutions as per the method for preparation of test solution in section 2.2.1. The resulting solutions were determined according to the method in the Chinese Pharmacopeia, and recovery was calculated. Average recovery was 99.56%, with RSD = 1.14% (n = 6).
Reproducibility test
Sophora alopecuroides L. medicinal materials of the same batch were prepared into six parallel test solutions as per the method in section 2.2.1, and quantitatively determined, respectively. Average content in that batch of medicinal materials was 0.214%, with RSD of 1.56% (n = 6), indicating good reproducibility of the present method.
Sample determination
Six batches of Sophora alopecuroides L. medicinal materials were determined according to the method described above. The results are shown in Table 1, which showed that matrine content differed rather greatly among Sophora alopecuroides L. of different habitat
Table I. Antibiotics susceptibility profile of the bacterial isolates. Aztreonam (ATM-30), cefotaxime (CTX-30), Aztreonam (ATM-30), cefotaxime (CTX-30), cefpodoxime (CPD-10), ceftriazone (CRO-30), ceftazidime (CAZ-30), ampicillin (AMP-10), penicillin (P-1), oxacillin (OX-1), piperacillin (PRL-75), imipenem (IPM-10), meropenem (MEM-10), norfloxacin (NOR-10), gentamicin (CN-10), and tetracycline (TE-30)
Inhibitory effect of matrine on growth of breast cancer MCF-7 cells
Cell culturing
MCF-7 cells were cultured routinely in RPMI 1640 medium containing 100 mL/L FBS, 100 ku/L penicillin and 100 mg/L streptomycin, and passaged in a 37? incubator containing 50 mL/L CO2. Medium was replaced every 2-3 days.
Inhibitory effect of matrine on proliferation of breast cancer MCF-7 cells
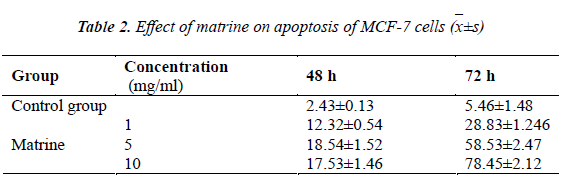
Logarithmic phase MCF-7 cells were collected, digested, centrifuged for 5 min, and then seeded in a 96-well culture plate at 5×105 cells/mL and 100 μL/well. After culturing for 24 h, different mass concentrations of matrine test solutions (1 mg/ml, 5 mg/ml and 10 mg/ml) were added, and control group was added with an equivalent amount of culture medium. Five parallel wells were set up for each group. After culturing for 72 h, 20 μL of MTT was added to each well, and the cultivation was continued for an additional 4 h. Then, supernatant was discarded, and each well was added with 100 μL of DMSO, and shaken gently. After dissolving approximately for 10 min, absorbance (A) value was measured at 490 nm using a microplate reader. Experimental results showed that the test concentrations of matrine could effectively inhibit the viability of MCF-7 cells and promote their apoptosis.
Compared with the control group, cell inhibitory effect of low concentration matrine increased gradually after acting for 48 h, and the MCF-7 cell inhibitory effect of high concentration matrine reached 78.45% after 72 h. Within the experimental range, MCF-7 cell growth inhibition rate of matrine increased with increasing drug concentration, showing a dose-response relationship.
Detection of apoptosis
MCF-7 cells cultured for 48 h in the Sophora alopecuroides L. treatment and control groups were collected, respectively. After adjusting cell concentration to 5 ×105 cells, each 100 μl was taken, and washed twice with PBS. Next, the cells were mixed well with 0.2 ml of PBS, transferred into a flow cytometry tube, centrifuged twice, added successively with 5 μl of Annexin V/FITC and 10 μl of propidium iodide (PI) stain, mixed well, and then kept in a dark place for 30 min. Finally, the cells were passed through a 300-mesh nylon net six times, and detected by flow cytometry to analyze the changes in cell cycle after treatment.
The results showed that after treatment with test concentrations of matrine for 48 h, cell cycle of MCF-7 cells could be markedly altered. With the addition of matrine, S phase MCF-7 cells were markedly reduced, and G0/G1 phase cells markedly increased, while G2/M phase cells did not change much. This suggested that matrine could change the MCF-7 cell cycle distribution, and arrest MCF-7 cells in G0/G1 phase, so that the cellular differentiation and proliferation rates were significantly inhibited.
Detection of effect of matrine on Bax, Bcl-2 protein expressions in MCF-7 cells
Logarithmic phase MCF-7 cells were randomly selected, treated with test concentrations of matrine for 48 h, and then fixed with 0.5% paraformaldehyde. Control group was added with the same amount of culture medium.
Cells in each treatment group were washed and centrifuged Then, supernatants were discarded, and each tube was added separately with mouse anti-human Bcl-2 and anti-Bax, mixed well, and incubated at 37? for 1 h, then washed, centrifuged, and supernatants were discarded. Next, each tube was added separately with 50 μL of FITC-labeled mouse anti-rabbit IgG (secondary antibody), and incubated at 37? for 30 min, then washed, centrifuged, and supernatants were discarded. Finally, the cells were washed with PBS solution, and analyzed by FACS, followed by recording of percentages of positive cells
Experimental results showed that compared with the control group, positive rate of Bax expression somewhat increased in various matrine concentration groups, which exhibited an increasing trend with increasing drug concentration. Expression of Bcl-2, on the contrary, decreased markedly in various treatment groups as compared with the control group, showing a negative correlation. See Table 4.
Discussion
Apoptosis is a process of autonomous cell death controlled by genes, which is a normal physiological phenomenon, and is one of important mechanisms for maintaining homeostasis; it often occurs in embryonic development and maintenance of tissue equilibrium. Necrosis and apoptosis are two completely different concepts, which differ essentially in terms of morphology and cellular biological properties. Apoptotic program possesses some special morphological features, including changes in plasma membrane, cytoplasmic and nuclear condensation, nuclear DNA cleavage, eventual formation of apoptotic bodies, and phagocytosis by macrophages. Necrosis is usually caused by cell damage or cytotoxic effect, where the release of necrotic cytoplasmic contents (mostly proteolytic enzymes) can cause inflammation of surrounding tissues.
Mechanism of apoptosis is not fully understood yet. Occurrence and development of apoptosis generally consist of three stages. The first stage is induction phase: after apoptosis-inducing factors acted on the cells, signals are delivered into cells through complex signaling pathways, and cells decide survival or death. The second stage is execution phase: cells decided to die initiate apoptosis according to preset program, activate various enzymes and degradation related substances required in apoptosis to form apoptotic bodies. The third stage is extinction phase: apoptotic cells are engulfed by neighboring cells, and degraded within phagocytes.
Apoptosis is a process tightly controlled by multiple genes. These genes are highly conserved between species, such as Bcl-2 family, caspase family, oncogenes like C-myc, and tumor suppressor gene P53. Bcl-2 family consists of two categories: apoptosis inhibiting genes like bcl-2, bcl-XL, bcl-w, A1/Bf1-1, Nr13 and mcL-1; and apoptosis promoting genes such as bax, Bik, Bad, bid, Hrk, bcl-Xs, bim and mtd. They can both promote and inhibit apoptosis, jointly maintaining the body's equilibrium. Bax can be involved in apoptosis; when inducing apoptosis, it moves from cytosol to the mitochondria and nuclear membranes. Bcl-2, on the other hand, inhibits apoptosis mainly through interaction with Bcl-2 family proteins. The two can form homodimers, or exist in the form of heterodimers.
As to the apoptosis influencing mechanism of Bcl-2 family, it is generally believed that bcl-2 and Bax influence the process of apoptosis by maintaining a certain dynamic proportion. Bcl-2 is the first gene confirmed to inhibit apoptosis, which is widely present in hematopoietic cells, epithelial cells, lymphocytes and cancer cells. High expression of bcl-2 maintains the mitochondrial calcium homeostasis, prevents the decline of mitochondrial membrane potential and the opening of mitochondrial permeability transition pores, inhibits the mitochondrial release of pro-apoptotic proteins, avoids the cascade reaction, and prevents apoptosis. Bax (Bcl-2 associated X protein) has the pore forming ability, and can induce the release of cytochrome c, activate Caspase-9, and trigger apoptotic cascade.
The results of this study show that 48 h after treatment of MCF-7 cells with different concentrations of matmatrine, a Sophora alopecuroides L. extract, positive rates of Bax expression in the treatment groups are elevated compared with the control group, while Bcl-2 expressions are markedly reduced. Moreover, in the treatment groups, the higher the concentration, the more evident the Bax and Bcl-2 influencing effect. Changes in the concentration of Bax and Bcl-2 indicate that matrine can influence the expression of Bax and Bcl-2 proteins in MCF-7 cells. Its mechanism of action needs further study.
References
- Qi YR, He SH, Shi GL. Research progress ofSophoraalopecuroidesL. Gansu Animal and Veterinary Sciences 2008; 38: 36-38.
- Jiangsu New Medical College. Dictionary of Chinese MateriaMediaca.Second Edition. Shanghai: Shanghai Scientific & Technical Publishers 2006: 1293- 1294.
- Negrete R, Cassels BK, Eckhardt G. (+)-9A-Hydroxymatrine from Sophoramacrocarpa. Phytochemistry1983; 22: 2069-2072.
- Zhang LZ, Li JS, Peter H. Studies on alkaloids in seeds of SophoraalopecuroidesL..China Journal of Chinese MateriaMedica 1997; 22: 740-743.
- Qin XG, Yuan YJ, Wu JC.Enhanced extraction of alkaloids from SophoraalopecuroidesL. by ion exchange at reduced pressure. J ChemEngJpn 2004; 37: 106-108.
- Ye G, Ma CH, Huang XY, Li ZX, Wang CG. Components of Sophoraalopecuroides seeds.Chem Nat Comp 2009; 45: 545-546.
- AttaUrRahman A, Choudhary MI, Parvez K, Ahmed A, Akhtar F, NurEAlam M, Hassan NM. Quinolizidinealkaloids from Sophoraalopecuroides. J Nat Prod 2000; 63: 190-192.
- Lu ZG, Hou YH, Lu NQ, Peng XD. The Epileptic Seizure-like Effect of Sophora Alkaloid Sophoridine on Experimental Animals. Journal of Ningxia Medical University 2009; 31: 723-725, 729.
- Ren D. Research, development and clinical application of compoundSophora composite membrane for oral ulcers. Chinese Traditional Patent Medicine 2000; 22: 590-591.
- Yu YQ, Ding PL, Chen DF.Determination of quinolizidinealkaloids in Sophora medicinal plants by capillary electrophoresis. Anal ChimActa 2004; 523: 15-20.
- Jiao HL, Yao R, Deng HZ, Wang XJ, Yuan HX, Yao H. Effect of Total Alkaloid of Sophoraalopecuroideson SW480 Cells and Balb/c Nude Mice Tumor Xenograft. Journal of Chinese Medicinal Materials 2011; 34: 1090-1093.
- Zhou Xu, NieLihong, Ding Juan, Zhou YZ. Effect of Kudouzi on contractile activity of smooth muscle in guinea pigs. Chinese Journal of Clinical Rehabilitation 2006; 10: 118-120.
- Hong G, Liu PX, Shen X. Inhibition of SophoraAlopecuoride Alkaloid Derivative SPRIDA on H22 Liver Cancer of Mice. Herald of Medicine 2008; 27: 369-371.
- Zhao JF, Li XM, Liu ST, Wang HB, Sun Q. Effects of matrine on related protein expression of cell proliferating and apoptosis in Tca-8113 cell line. Medical Innovation of China 2009; 6: 1-3.
- Yang HW, Ai L, Luo QJ. In vitro Experiment of Inhibiting Bacteria of SophoraalopecuroidesL. and Other Xinjiang Medicinal Plants. Journal of Anhui Agricultural Sciences 2008; 36: 12745-12746.