ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 12
MMP-2 expression and MVD in invasive cervical carcinoma
Chun-Zhi Yu* and Xian-Li Zhao
Department of Gynaecology and Obstetrics, Northwest Women’s and Children’s Hospital, Xi’an, Shaanxi, PR China
- *Corresponding Author:
- Chun-Zhi Yu
Department of Gynaecology and Obstetrics
Northwest Women’s and Children’s Hospital, PR China
Accepted on April 17, 2017
Objective: This study aims to investigate Matrix Metalloproteinase-2 (MMP-2) expression and Microvessel Density (MVD) in invasive cervical cancer and determine the malignant degrees of cervical adenocarcinoma and squamous carcinoma through protein level detection.
Methods: Fifty cases of patients with cervical squamous carcinoma and 40 cases of patients with cervical adenocarcinoma were recruited from 2010 to 2017. MMP-2 protein expression and MVD in the tumor sections were analysed using immunohistochemistry method.
Results: MVD in patients with cervical adenocarcinoma was higher than that in patients with squamous carcinoma (P<0.05). MMP-2 expression in patients with cervical squamous carcinoma (+++) was significantly higher than that in patients with cervical adenocarcinoma (P<0.001). Analysis of the clinicpathological features indicated that MMP-2 expression differed between patients with cervical squamous carcinoma and adenocarcinoma. The positive value of MVD in squamous carcinoma stromal cells was significantly higher than that in cancer cells (P<0.05). MMP-2 was positively expressed at similar intensities in the stromal cells and cancer cells of patients with cervical adenocarcinoma (P>0.05).
Conclusion: The high MMP-2 expression and MVD in patients with cervical adenocarcinoma indicated that the malignant degree of adenocarcinoma was higher than that of squamous carcinoma. The findings indicated that the patients underwent tumor angiogenesis, invasion, or metastasis and thus should be further treated.
Keywords
Cervical cancer, Human matrix metalloproteinase-2, Microvessel density, Immunohistochemistry.
Introduction
Tumor vessels can provide nutrients and a metastasis pathway for tumor growth, metastasis, and infiltration. Tumor cells can secrete various cytokines that promote tumor angiogenesis [1]. Cyclooxygenase (COX) is a key enzyme that catalyses the synthesis of prostate-like substances, including COX-1 and COX-2 subtypes. Studies indicated the close relationship between the high expression of COX-2 and cervical cancer. In particular, COX-2 plays an important role in the local angiogenesis, invasion, and metastasis of cervical cancer [2,3]. Moreover, the expression of COX-2 is positively correlated with that of Matrix Metalloproteinase-2 (MMP-2). The higher the expression of COX-2 is, the higher the in vivo activity of MMP-2 will be [4]. Once the COX-2 pathway is blocked, the MMP-2 function will also be suppressed. Therefore, biological function can be achieved by inhibiting COX-2 expression and blocking the MMP-2 pathway. In the present study, the expression levels of COX-2 and MMP-2 in patients with cervical cancer were investigated through immunohistochemistry. The Microvessel Density (MVD) of neovascular endothelial cells was also calculated through CD105. Results may provide a basis for using COX-2,MMP-2, and MVD to detect early angiogenesis, progression, and prognosis of cervical cancer.
Materials and Methods
General information
Fifty patients with cervical squamous carcinoma and 40 patients with cervical adenocarcinoma were recruited from our hospital from 2010 to 2017. All the patients did not undergo immunotherapy, chemotherapy, or radiotherapy before the operation. Patients with squamous carcinoma, aged 32-80 years (average age of 51.3 ± 2.9 y), were categorized into different clinical stages based on the criteria revised by the International Federation of Gynecology and Obstetrics (FIGO): stage Ib (n=12), stage IIa (n=12), stage IIb (n=15), stage III (n=2), and stage IV (n=4). Based on the histological cell grading of squamous carcinoma, the tumors were classified as grade I (n=7), grade II (n=25), and grade III (n=18); of these cases, 16 were accompanied by lymph node metastasis. Patients with adenocarcinoma, aged 29-76 y (average age of 48.5 ± 3.7 y), are categorized based on clinical stage into stage Ib (n=14), stage IIa (n=14), stage IIb (n=14), and stage III (n=4). Based on the histological cell grading of adenocarcinoma, the tumors were classified into grade I (n=16), grade II (n=16), and grade III (n=8); of these cases, 10 were accompanied by lymph node metastasis. Age, clinical stage, histological grade, and lymph node metastasis were not significantly different the two groups of patients (P>0.05).
Method
Immunohistochemistry SP staining was performed using kits provided by Fuzhou Maixin Biotechnology Development Co., Ltd. and MMP-2, MMP-9, TIMP-1, and TIMP-2 as monoclonal antibodies. Experimental operations were conducted in strict accordance with the kit instructions. The primary antibody in the blank control group was replaced by PBS (phosphate buffer). Cervical cancer tissues were used as positive control.
Result criteria
MVD count: The area with the highest MVD in tumor tissues was detected at low magnification. Microvessels were counted in three visual fields at 200X magnification.
Expression of MMP-2: Semi-quantitative analysis was conducted according to Fomwitz comprehensive scoring method. The brown granule cells in the cytoplasm observed under optical microscope were used as positive control. Five high power fields (400X) were randomly selected for analysis. Both tumor and positive cells were counted. The percentage of positive cells was also calculated. Staining intensity was derived from the staining score of multiple positive cells. Two scores were added eventually: (–) 0-1; (+): 2-3; 4-5 (++); (++ +): 6-7.
Statistical methods
Data were analysed using SPSS19.0 statistical software. MMP-2 expression was shown using n (%) and compared among the groups by using χ2 test. MVD was presented as χ ± s and compared among the groups by using t test. Differences at P<0.05 were considered statistically significant.
Results
MMP-2 expression and MVD in cervical squamous carcinoma and adenocarcinoma
MMP-2 expression (+++) in patients with squamous carcinoma was significantly higher than that in patients with adenocarcinoma. However, MMP-2 expression (+) in patients with squamous carcinoma was significantly lower than that in patients with adenocarcinoma. The differences were statistically significant (P<0.001, Table 1).
| Group | n | MMP-2 | MVD | |||
|---|---|---|---|---|---|---|
| – | + | ++ | +++ | |||
| Squamous carcinoma | 50 | 8 | 8 | 12 | 17 | 46.78 ± 18.25 |
| Adenocarcinoma | 40 | 6 | 24 | 10 | 0 | 63.50 ± 28.32 |
| t value | 0.01 | 18.56 | 0.01 | 16.58 | 3.23 | |
| P value | 0.89 | <0.001 | 0.91 | <0.001 | 0.001 | |
Table 1. MMP-2 expression and MVD in patients with cervical squamous carcinoma and adenocarcinoma (n; χ ± s).
Relationship of clinico-pathological features to MMP-2 expression and MVD in patients with cervical squamous carcinoma and adenocarcinoma
Compared with patients with cervical squamous carcinoma (▲P<0.05 and P>0.05, Table 2).
| Condition | n | MMP-2 | MVD (/200 times) | ||||
|---|---|---|---|---|---|---|---|
| – | + | ++ | +++ | ||||
| Clinical stage Ib-IIa | Squamous carcinoma | 27 | 6 | 6 | 6 | 9 | 44.75 ± 18.43 |
| Adenocarcinoma | 28 | 2 | 16 | 10 | 0▲ | 68.22 ± 30.14▲ | |
| Clinical stage IIb-IV | Squamous carcinoma | 23 | 3 | 4 | 7 | 9 | 49.26 ± 18.24 |
| Adenocarcinoma | 12 | 4 | 8 | 0 | 0▲ | 52.50 ± 21.89△ | |
| Differentiated degree I-II | Squamous carcinoma | 31 | 3 | 5 | 10 | 13 | 45.10 ± 15.12 |
| Adenocarcinoma | 32 | 4 | 18 | 10 | 0▲ | 67.46 ± 29.40▲ | |
| Differentiated degree III | Squamous carcinoma | 19 | 6 | 4 | 3 | 6 | 49.88 ± 23.31 |
| Adenocarcinoma | 8 | 2 | 6 | 0 | 0▲ | 46.67 ± 18.44△ | |
| Lymphatic metastasis | Squamous carcinoma | 16 | 0 | 3 | 4 | 9 | 57.95 ± 5.68 |
| Positive | Adenocarcinoma | 10 | 2 | 6 | 2 | 0▲ | 81.73 ± 41.07▲ |
| Lymphatic metastasis negative | Squamous carcinoma | 34 | 8 | 7 | 9 | 10 | 42.54 ± 19.62 |
| Adenocarcinoma | 30 | 4 | 18 | 8 | 0▲ | 57.42 ± 21.18▲ | |
Table 2. Relationship of clinico-pathological features to MMP-2 and MVD (n; χ ± s) in patients with cervical squamous carcinoma and adenocarcinoma.
MMP-2 expression in different cervical tissues
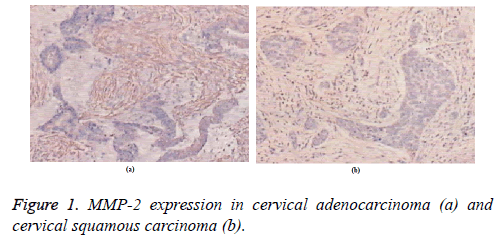
MMP-2 was mainly expressed in the cytoplasm and in the cell membrane but not expressed in the nucleus. MMP-2 was expressed at similar intensities in both stromal and cancer cells of patients with cervical adenocarcinoma (Figure 1a). Cancer cells in lymph node metastasis showed homogeneous staining, whereas lymphocytes were not stained. MMP-2 was not expressed in non-lymph node metastasis. In patients with cervical squamous carcinoma, MMP-2 mainly embraced the tumor stromal cells but was scattered in the cancer nests. MMP-2 expression in the cancer edge was relatively strong (Figure 1b). The positive expression level of MMP-2 in squamous carcinoma was significantly higher than that in cancer cells.
Discussion
In metastasis, tumor cells can pass through a series of barriers composed of extracellular matrix and basilar membrane. The cell matrix and collagen can be degraded by MMPs after local dissolution to form a tumor metastasis pathway. MMPs are important proteolytic enzymes in the body [5]. Of the 26 MMP subtypes discovered, MMP-2 plays an important role in the infiltration and metastasis of the whole tumor because of the following mechanisms. First, MMP-2 can degrade the extracellular matrix to provide adequate space for growth of blood vessels and release angiogenesis factors stored in the matrix. Second, MMP-2 can regulate, synthesize, and release angiogenesis factors [6,7]. MMP-2 can also strengthen the vascular signal system and promote the neogenesis of blood capillary by activating vascular cell surface receptors. The invasion and metastasis of tumors cannot occur without the degradation of extracellular matrix. The degradation process relies on proteolytic enzymes [8]. MMP-2 is secreted in the form of zymogen. Activation of zymogen produces type IV collagenase, which destroys and degrades the extracellular matrix and basilar membrane on the tumor surface. In the present study, the more positive the expression of MMP-2 is, the more prominent the lymph node metastasis will be.
Cervical cancer is a type of solid tumor. Early solid tumor grows slowly until it reaches 1-2 mm2 (about 106 cells); thereafter, the growth is terminated. Only the growth of vascularized tumors is of certain clinical significance. CD34 is a protein substance that can be used as marker for vascular endothelial cells and for quantitative analysis of MVD [9]. Endothelial cells possess cracks in the wall because of immature microvascular growth. Tumor cells can easily pass through the microvessels and undergo distant metastasis through blood circulation after fibrinolytic reaction lacking endothelial cells and basilar membrane. Studies reported that the higher the MVD is, the higher the malignant degree and the more rapid the tumor growth and metastasis will be. MVD in patients with cervical adenocarcinoma was positively correlated with the expression in ascite cancer cells. MVD in patients with cervical adenocarcinoma was higher than that in patients with cervical squamous carcinoma [10]. This finding indicates the formation of numerous new vessels in patients with cervical adenocarcinoma, leading to high malignant degree. Moreover, the clinical stages Ib-IIa, pathological differentiation grade, lymph node metastasis, negative lymph node metastasis, and positive lymph node metastasis in patients with adenocarcinoma were significantly higher than those in patients with squamous carcinoma (P<0.05). Hence, the malignant degree of patients with adenocarcinoma was higher and more related to the high expression of MVD even in the same pathological states. The clinical stage Ib-IV and the pathological differentiation grade III were not significantly different between patients with squamous carcinoma and cervical adenocarcinoma. The lack of significant differences could be related to the small sample size employed in the study.
Conclusion
Monitoring MMP-2 expression in patients with cervical cancer can effectively detect cancer cell invasion and metastasis. MMP-2 expression can be used to identify the malignant degree and prognosis of cervical cancer. Meanwhile, monitoring MVD in patients can effectively determine the distant metastasis in patients with cervical adenocarcinoma.
References
- Liu L, Ke F, Li C, Zhang X, Pi C, Wang L, Ke S, Luo Y, Zhong Z. Multivesicular liposomes loading with ursolic acid enhanced the in vitro antitumor activity on hepatic carcinoma cells. Lat Am J Pharm 2017; 36: 758-766.
- Kalem F, Durmaz S, Ozdemir B, Ergun AG, Ertugrul O. The diagnostic value of procalcitonin, WBC and CRP in diagnosis of lower respiratory tract infections in elderly patients. Biomed Res India 2017; 28: 1012-1015.
- Boss EA, Barentsz JO, Massuger LFAG, Boonstra H. The role of MR imaging in invasive cervical carcinoma. Eur Radiol 2000; 10: 256-270.
- Hu N, Hu Y, Zhu XF. Synthesis and evaluation of tetrahydroisoquinoline derivatives against human hepatoma carcinoma cell lines. Lat Am J Pharm 2017; 36: 192-195.
- Zehbe I, Wilander E, Delius H, Tommasino M. Human papillomavirus 16 E6 variants are more prevalent in invasive cervical carcinoma than the prototype. Cancer Res 1998; 58: 829-833.
- Moberg M, Gustavsson I, Wilander E, Gyllensten U. High viral loads of human papillomavirus predict risk of invasive cervical carcinoma. Brit J Cancer 2005; 92: 891-894.
- Bai X, Guan B, Liu M, Zhu Q, He Y, Wang P, Wang Y, Li Q. The antitumor effect of hederagenin on tumors growth of hepatocarcinoma (H22) tumor-bearing mice. Lat Am J Pharm 2017; 36: 142-150.
- Yin Y, Xu Y, Ma H, Tian X. Hesperetin ameliorates cardiac inflammation and cardiac fibrosis in streptozotocin-induced diabetic rats by inhibiting NF-κB signaling pathway. Biomed Res India 2017; 28: 223-229.
- Hricak H, Powell CB, Yu KK, Washington E, Subak LL. Invasive cervical carcinoma: role of MR imaging in pretreatment work-up-cost minimization and diagnostic efficacy analysis. Radiology 1996; 198: 403-409.
- Vanitha L, Suresh GR, Chandrasekar M, Punita P. Development of four stress levels in group stroop colour word test using HRV analysis. Biomed Res India 2017; 28: 98-105.