ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 1
Neuroprotection of lurasidone by antagonist activities of histamine in a cranial nerve involvement mouse model
Lurasidone is a novel antipsychotic agent approved for the treatment of schizophrenia and various mental diseases in many countries. In this study, neuroprotection of Lurasidone by antagonist activities of histamine was investigated in a cranial nerve involvement mouse model. The antagonist activities of Lurasidone for serotonin 5-HT7, serotonin 5-HT2A, serotonin 5-HT1A and dopamine D2 were analyzed and the preclinical therapeutic effects of Lurasidone were studied in a cranial nerve involvement mouse model. This study also assessed safety, maximum tolerated dose (MTD), and preliminary protective activity of Lurasidone in a cranial nerve involvement mouse model. The therapeutic dose was 0.14 mg once daily administered continuously in 30-day cycles. Our results found that our clinical prescriptions induced positive behavioral responses after treatment with Lurasidone. MTD was identified as once daily Lurasidone 0.32 mg. The most common treatment-related treatment-emergent adverse events were proteinuria 30% and nausea 40%. Long-term treatment of Lurasidone for cranial nerve involvement has been shown to better therapeutic effects and reduced anxiety of experimental mice. In addition, treatment of Lurasidone did not affect body weight. Expression of Foxp2 and Neuroglobin was increased and the neuroprotective protein SxIP and EB were decreased in cortical astrocytes cells of the schizophrenia mice after Lurasidone treatment. Lurasidone therapy showed a reinforced memory capability and anxiety decreasing. In conclusion, the Lurasidone treatment may protect against language disturbances associated with negative and cognitive impairments in a cranial nerve involvement mouse model, which provide a basis for clinical treatment of patients with cranial nerve involvement.
Keywords
Lurasidone, Neuroprotection, Cranial nerve involvement, Antagonist
Introduction
Schizophrenia is one of the most common mental disorder diseases that impacts approximately more than one percent of the adult population all over the world [1]. Patients with schizophrenia often suffer from perplexing psychiatric with cognitive, language, memory and behavior impairment [2,3]. Moreover, children patients with schizophrenia often have to drop out from school and receive poor education due to the often chronic course of illness, which further reduce the quality of life, impairment in independent living and major sociooccupational dysfunction [4]. Furthermore, cognitive impairment is the key characterization of schizophrenia and the neuroprotective agents for neurocognitive treatment have been in research and development state according to cognitive deficits across multiple domains in substantial intellectual impairment [5,6]. Though the negative influences of schizophrenia for human life and social order are well understood, the treatment of this kind of disease is still remaining less optimistic and mechanism of these treatments are required to be clearly elaborated [7].
In recent years, the function of neuroprotection factors for schizophrenia study has been valued for increasing interest. In addition, neuroprotection agents have developed a therapeutic strategy to prevent deterioration associated with schizophrenia and repair the damaged neurons with the purpose of treatment [8]. Furthermore, previous study has evidenced that schizophrenia patients converted to psychosis were found to associate with progressive gray matter loss in the intracranial cortex, which suggests neuroprotection possesses the maintenance of the functional integrity of the brain in response to neurobiological stress and could protects neuron against nerve injury [9]. Therefore, neuroprotective agents were not only beneficial to understand the mechanisms of schizophrenia but also improve outcomes for patients with schizophrenia.
Histamine is essential for occurrence, development and deterioration of schizophrenia that has become a focus in the treatment of mental disorder diseases and antipsychotic agents are following come out as medical development. Additionally, Lurasidone also was identified as a therapy with lithium or valproate for the treatment of bipolar I depression [10]. The key therapeutic effects of Lurasidone are depended on antagonist activities of for serotonin 5-HT2A, serotonin 5- HT1A, serotonin 5-HT7 and dopamine D2 [11]. Furthermore, the efficacy and safety of Lurasidone for schizophrenia therapy has been well understood from clinical trials [11]. However, neuroprotection of Lurasidone for patients with schizophrenia is not clearly understood.
In this present study, we investigated the efficacy of Lurasidone in a schizophrenia mouse model. On the basis of the aforementioned evidence, our results further explored the preclinical efficacy and beneficial outcomes of Lurasidone. Our date showed that Lurasidone down-regulated 5-HT2A, 5- HT1A, 5-HT7, D2 and extended overall survival of schizophrenia mice compared with PBS-treated mice. This study also suggests that cognitive ability were improved after treatment with Lurasidone.
Methods
Analysis of histamine expression
We analyzed 5-HT2A, 5-HT1A, 5-HT7 and D2 in the cerebrospinal fluid in mice with schizophrenia by using commercialized Mouse ELISA Kit. The ELISA assays were performed according to the manufacturer’s protocols. The result was measured at 450 nm in an ELISA reader and finally conversed to finial concentrations.
Real-Time quantitative PCR (RT-qPCR)
Total RNA was extracted from mice cortical astrocytes cells by using RNAeasy Mini Kit (QIAGEN, Gaithersburg, MD) and subjected to transcribe into cDNA by using the reverse transcription kit (QIAGEN, Gaithersburg, MD). The cDNA (10 ng) was used for real-time quantitative PCR (Bio-Rad, MD, USA). Relative mRNA expression changes were calculated by 2−ΔΔCt. The results are expressed as the n-fold way compared to control.
Lurasidone treatment effects on mice with schizophrenia
Six to eight week old BALB/c was purchased and induced by methionine to form schizophrenia model. The schizophrenia mice were randomized into two groups and injected intravenously with Lurasidone (0.32 mg) as PBS as control. The total therapeutic times were 14 times once daily. All experimental mice procedures were performed and approved according to the guidelines of Animal Care and Use Committee of Tianjin medical university.
Experiment of elevated plus maze
Schizophrenia mice were purchased and feed in diurnal cycle environment for our design analysis. Anxiety of mice was evaluated by the elevated plus maze trial based on the hypothesis that mice bearded fear of open field. The details of elevated plus maze trial were descripted in previous study and the space was amplified three times in a size of 100 cm × 20 cm × 80 cm [12]. The experimental mice with schizophrenia were placed into the center of the elevated plus maze. The schizophrenia mice were made to face an open arm for a total five minutes. The time spent in the open and closed arms was recorded and calculated by employing the formula: D2 = (B - A)/ (B + A). A represented the time spent in open arm and the B represented the time spent in closed arm. The anxious behavior was measured by above formula.
Statistical methods
All data were present as means and SEM of triplicate. Unpaired data was determined by Student’s t test and comparisons of data between multiple groups were analysed by variance (ANOVA). Kaplan-Meier was used to estimate the risk of relapse and re-treatment during 368-day treatment. *P<0.05 and **P<0.01 was considered statistically significant.
Results and Discussion
Treatment duration, Dose-limiting toxicities and maximum tolerated dose of Lurasidone
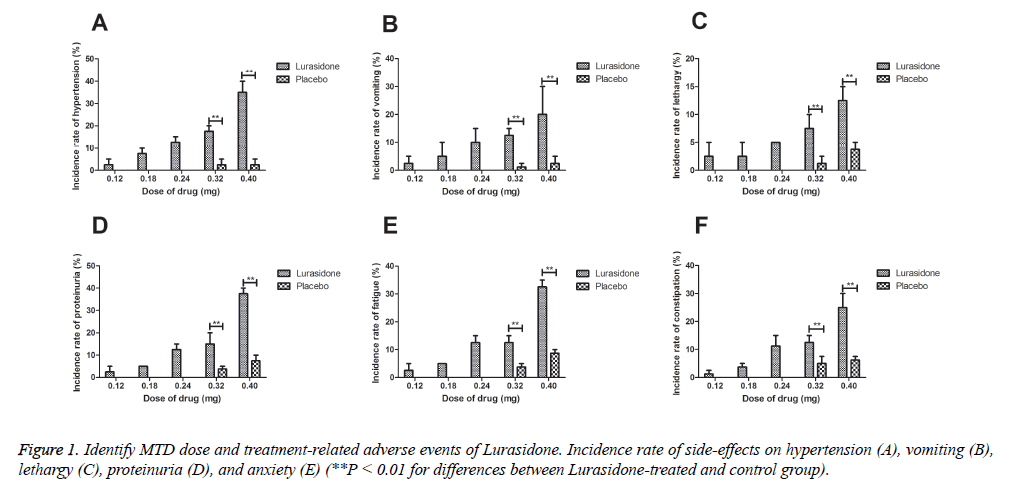
A novel antipsychotic agent Lurasidone was approved for treatment of patients with schizophrenia and it was identified as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression [10]. In this study, Lurasidone was selected to investigate neuroprotection. Treatment duration, Dose-limiting toxicities and maximum tolerated dose are essential for drugs in clinical application [13]. Therefore, median overall treatment duration was identified as 30 days and across dosing cohorts were 0.12, 0.18, 0.24, 0.32 and 0.40 mg. In this study, 0.32 mg of Lurasidone once daily was identified as the maximum tolerated dose (MTD). All the experimental mice were received Lurasidone once daily. We observed the most common treatment-related adverse events were hypertension, vomiting, lethargy, proteinuria, fatigue and constipation (Figures 1A-1E).
Detection of histamine changes in cerebrospinal fluid cranial in nerve involvement mouse model
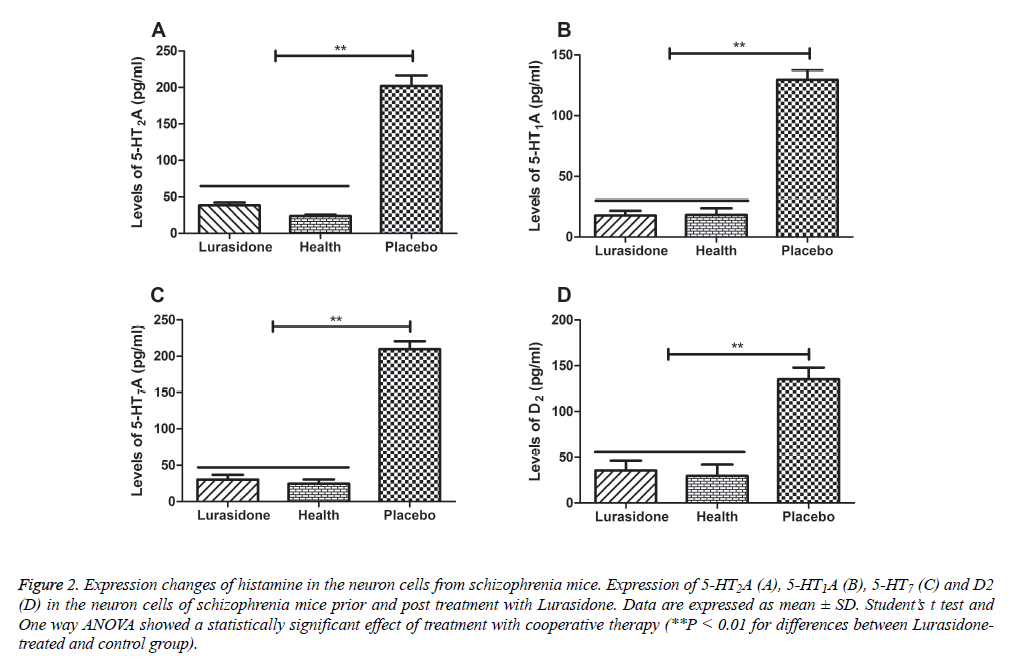
Schizophrenia is well known to be related with neuronal dysfunction and neuroprotection is beneficial for schizophrenia therapy in microenvironment in brain [14]. Lurasidone is similar to the most antipsychotic drugs that possesses antagonist at serotonin 5-HT7, serotonin 5-HT2A, serotonin 5- HT1A and dopamine D2, respectively [15]. Subsequently, histamine changes were detected in the cerebrospinal fluid in mice with schizophrenia prior and post treatment with Lurasidone. Our results in Figures 2A-2D showed that the concentration of 5-HT2A, 5-HT1A, 5-HT7 and D2 was elevated in mice with chronic schizophrenia compared with healthy mice. However, histamine levels in chronic schizophrenia mice were decreased after Lurasidone treatment. Our date indicated that 5-HT2A, 5-HT1A, 5-HT7 and D2 concentration was recovered to normal levels after treatment with Lurasidone compared to healthy mice.
Figure 2: Expression changes of histamine in the neuron cells from schizophrenia mice. Expression of 5-HT2A (A), 5-HT1A (B), 5-HT7 (C) and D2 (D) in the neuron cells of schizophrenia mice prior and post treatment with Lurasidone. Data are expressed as mean ± SD. Student’s t test and One way ANOVA showed a statistically significant effect of treatment with cooperative therapy (**P < 0.01 for differences between Lurasidonetreated and control group).
Therapeutic effects of Lurasidone on a schizophrenia mouse model
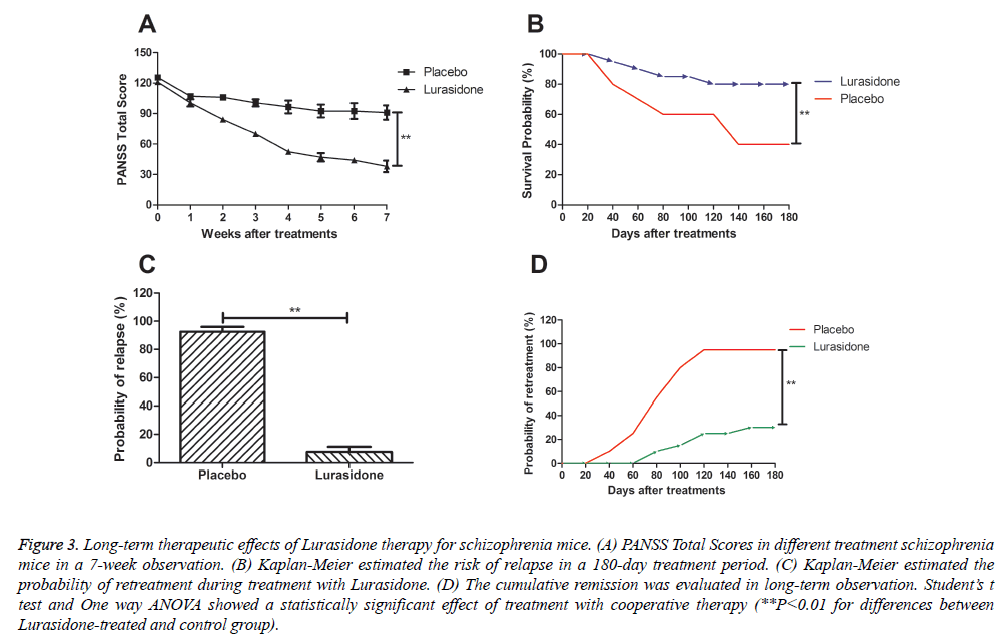
Mechanism studies upon schizophrenia have indicated that PANSS total score are height correlation with the progress of schizophrenia [16-19]. The result in Figure 3A showed that PANSS total score was lower in Lurasidone-treated cranial nerve involvement mice compared to control group. From our date we demonstrated that PANSS score was 38.45 ± 7.55, while control-treated mice presented a higher PANSS score. In addition, reports have shown that long-term survival and relapse rate are potential detection index for patients with schizophrenia [20-22]. The results in Figure 3B showed that Lurasidone prolonged the survival of mice with schizophrenia based on 180-day preclinical observation. Furthermore, the result in Figure 3C showed a lower risk of relapse rate after Lurasidone treatment compared to control. Also, Figure 3D demonstrated that Lurasidone therapy significantly decreased the risk of re-treatment in 180-day observation and indicated that treatment significantly relieved higher rates of remission.
Figure 3: Long-term therapeutic effects of Lurasidone therapy for schizophrenia mice. (A) PANSS Total Scores in different treatment schizophrenia mice in a 7-week observation. (B) Kaplan-Meier estimated the risk of relapse in a 180-day treatment period. (C) Kaplan-Meier estimated the probability of retreatment during treatment with Lurasidone. (D) The cumulative remission was evaluated in long-term observation. Student’s t test and One way ANOVA showed a statistically significant effect of treatment with cooperative therapy (**P<0.01 for differences between Lurasidone-treated and control group).
Neuroprotection of Lurasidone on mice with schizophrenia
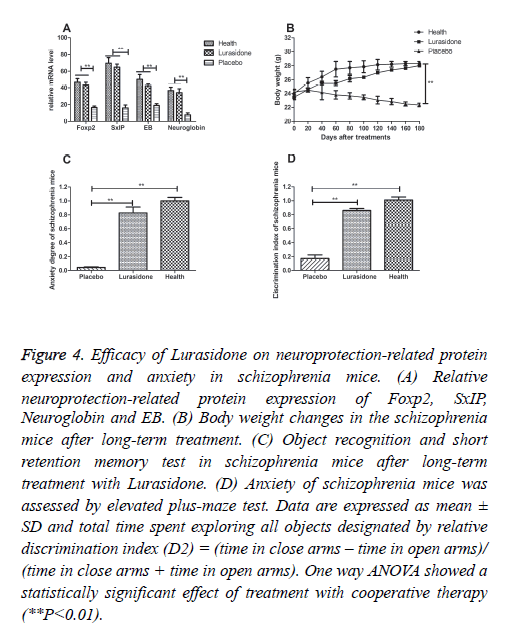
Neuroprotection-related protein expressions have been found to play an important role in the pathogenesis of schizophrenia [23]. Neuroprotective protein of Foxp2, SxIP, Neuroglobin and EB mRNA levels were analyzed in this work. Foxp2, SxIP, EB and Neuroglobin expressions were increased in the brain neuron from the schizophrenia mice after Lurasidone treatment (Figure 4A), which led to neuroprotection and synaptic plasticity. Moreover, we observed that treatment of Lurasidone slightly affected body weight, while placebo group mice showed a decreasing body weight (Figure 4B). In addition, anxiety is the most important characteristics for schizophrenia [24]. The behavior of schizophrenia mice was assessed by an elevated plus maze and recognition test. The anxiety behavior of schizophrenia mice was assessed by an elevated plus maze and recognition test. Our results in Figure 4C showed Lurasidone treatment relieved the anxiety degree of the schizophrenia mice (discrimination index-D2, 0.725 vs 0.041 **P=0.0062, respectively). Furthermore, the results in Figure 4D showed that discrimination index was 0.846 vs 0.161 in Lurasidone and control, respectively (**P=0.0031), which showed a reinforced memory capability after Lurasidone treatment compared to control.
Figure 4: Efficacy of Lurasidone on neuroprotection-related protein expression and anxiety in schizophrenia mice. (A) Relative neuroprotection-related protein expression of Foxp2, SxIP, Neuroglobin and EB. (B) Body weight changes in the schizophrenia mice after long-term treatment. (C) Object recognition and short retention memory test in schizophrenia mice after long-term treatment with Lurasidone. (D) Anxiety of schizophrenia mice was assessed by elevated plus-maze test. Data are expressed as mean ± SD and total time spent exploring all objects designated by relative discrimination index (D2) = (time in close arms – time in open arms)/ (time in close arms + time in open arms). One way ANOVA showed a statistically significant effect of treatment with cooperative therapy (**P<0.01).
In conclusion, the antagonist of Lurasidone for histamine receptors was confirmed and neuroprotection was found in mice with schizophrenia. Our date demonstrated the MTD of Lurasidone was 0.32 mg once daily and body weight was minimal affected in the treatment of schizophrenia mice. Taken together, Lurasidone represented a better efficacy for anxiety in schizophrenia mice with few side-effects for experimental mice.
References
- Wieland J, Zitman FG. Brief Symptom Inventory symptom profiles of outpatients with borderline intellectual functioning and major depressive disorder or posttraumatic stress disorder: Comparison with patients from regular mental health care and patients with Mild Intellectual Disabilities. Research in developmental disabilities 2016; 51-52: 153-159.
- Suzuki T. A further consideration on long-acting injectable versus oral antipsychotics in the treatment of schizophrenia: a narrative review and critical appraisal. Expert opinion on drug delivery 2015; 1-12.
- Bortolato B, Miskowiak KW, Kohler CA, Vieta E, Carvalho AF. Cognitive dysfunction in bipolar disorder and schizophrenia: a systematic review of meta-analyses. Neuropsychiatric disease and treatment 2015; 11: 3111-3125.
- Crowe M, Inder M, Beaglehole B. More than medication - providing interventions that target the complexities of mental disorder. Journal of psychiatric and mental health nursing 2016; 23: 1-2.
- O'Reilly K, Donohoe G, O'Sullivan D, Coyle C, Mullaney R, O'Connell P, Maddock C, Nulty A, O'Flynn P, O'Connell C, Kennedy HG. Study protocol: a randomised controlled trial of cognitive remediation for a national cohort of forensic mental health patients with schizophrenia or schizoaffective disorder. BMC psychiatry 2016; 16: 5.
- Korinek K, Loebach P, Teerawichitchainan B. Physical and Mental Health Consequences of War-related Stressors Among Older Adults: An Analysis of Posttraumatic Stress Disorder and Arthritis in Northern Vietnamese War Survivors. The journals of gerontology. Series B, Psychological sciences and social sciences 2016.
- Lurasidone for schizophrenia. Drug and therapeutics bulletin 2015; 53: 30-32.
- Lieberman JA, Perkins DO, Jarskog LF. Neuroprotection: a therapeutic strategy to prevent deterioration associated with schizophrenia. CNS spectrums 2007; 12: 1-13.
- Jarskog LF, Lieberman JA. Neuroprotection in schizophrenia. The Journal of clinical psychiatry 2006; 67: e09.
- Loebel A, Cucchiaro J, Silva R, Kroger H, Sarma K, Xu J, Calabrese JR. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. The American journal of psychiatry 2014; 171: 169-177.
- Citrome L. Lurasidone in schizophrenia: new information about dosage and place in therapy. Advances in therapy 2012; 29: 815-825.
- Vaisburd S, Shemer Z, Yeheskel A, Giladi E and Gozes I. Risperidone and NAP protect cognition and normalize gene expression in a schizophrenia mouse model. Scientific reports 2015; 5: 16300.
- Postel-Vinay S, Collette L, Paoletti X, Rizzo E, Massard C, Olmos D, Fowst C, Levy B, Mancini P, Lacombe D, Ivy P, Seymour L, Le Tourneau C,Siu LL, Kaye SB, Verweij J, Soria JC. Towards new methods for the determination of dose limiting toxicities and the assessment of the recommended dose for further studies of molecularly targeted agents--dose-Limiting Toxicity and Toxicity Assessment Recommendation Group for Early Trials of Targeted therapies, an European Organisation for Research and Treatment of Cancer-led study. Eur J Cancer 2014; 50: 2040-2049.
- Lieberman JA. Neuroprotection: a new strategy in the treatment of schizophrenia. Neurobiological basis of neurodegeneration and neuroprotection. CNS spectrums 2007; 12: 4-6.
- Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, Matsumoto K, Nishikawa H, Ueda Y, Toma S, Oki H, Tanno N, Saji I, Ito A, Ohno Y,Nakamura M. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. The Journal of pharmacology and experimental therapeutics 2010; 334: 171-181.
- Paul S, Sharfman N. Functional Connectivity as a Means to Delineate Differences between Treatment Resistant and Treatment Responsive Schizophrenia. Journal of neurophysiology: jn 01127 02015, 2016.
- Li JC, Zhang WJ, Zhu JX, Zhu N, Zhang HM, Wang X, Zhang J, Wang QQ. Preparation and brain delivery of nasal solid lipid nanoparticles of quetiapine fumarate in situ gel in rat model of schizophrenia. International journal of clinical and experimental medicine 2015; 8: 17590-17600.
- Yoshimura R, Hori H, Katsuki A, Atake K, Nakamura J: Serum levels of brain-derived neurotrophic factor (BDNF), proBDNF and plasma 3-methoxy-4-hydroxyphenylglycol levels in chronic schizophrenia. Annals of general psychiatry 2016; 15: 1.
- Genc A, Kalelioglu T, Karamustafalioglu N, Tasdemir A, Genc ES, Akkus M, Emul M. Serum soluble urokinase-type plasminogen activator receptor levels in male patients with acute exacerbation of schizophrenia. Psychiatry research 2016.
- Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry and clinical neurosciences 2010; 64: 217-230.
- de Witte L, Tomasik J, Schwarz E, Guest PC, Rahmoune H, Kahn RS, Bahn S. Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophrenia research 2014; 154: 23-29.
- Debnath M, Chaudhuri TK: The role of HLA-G in cytokine homeostasis during early pregnancy complicated with maternal infections: a novel etiopathological approach to the neurodevelopmental understanding of schizophrenia. Medical hypotheses 2006; 66: 286-293.
- Morrison BE, Majdzadeh N, Zhang X, Lyles A, Bassel-Duby R, Olson EN, D'Mello S. Neuroprotection by histone deacetylase-related protein. Molecular and cellular biology 2006; 26: 3550-3564.
- Goryunova AV, Danilova LY, Goryunov AV. Characteristics of neurological status in children with schizophrenia and schizotypal disorder. Zhurnal nevrologii i psikhiatrii imeni S.S. Korsakova / Ministerstvo zdravookhraneniia i meditsinskoi promyshlennosti Rossiiskoi Federatsii, Vserossiiskoe obshchestvo nevrologov [i] Vserossiiskoe obshchestvo psikhiat 2015; 115: 14-20.