ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2010) Volume 21, Issue 4
New Amides of Arachidonic-Acid as Potential Antiinflammatory Drugs: A Preliminary Study
Autore G2, Marzocco S2, Palladino C2, Saturnino C2, Sinicropi S1, Spagnuolo A2. Vivacqua E2, A. Capasso2*
1Department of Pharmaceutical Sciences, University of Calabria , Arcavacata di Rende, Cosenza, Italy
2Department of Pharmaceutical Sciences, University of Salerno, Fisciano (SA), Italy, Via Ponte Don Melillo 84084 Fisciano (SA) Italy
- *Corresponding Author:
- Anna Capasso
Department of Pharmaceutical Sciences
University of Salerno, Fisciano (SA)
Italy, Via Ponte Don Melillo 84084 Fisciano (SA)
Italy
Email: annacap@unisa.it
Accepted date: July 23 2010
The arachidonic acid is a precursor in the synthesis of eicosanoids: through the action of cyclooxygenase and peroxidase, it gives rise to prostaglandins then is converted into differ-ent substances involved in inflammation and in many physiological reactions as protectors of gastric mucosa. The aim of this research was to verify the pharmacological activity in a new series of amides of arachidonic acid. To this end, we have been synthesized new deriva-tives and performed cytotoxicity studies and preliminary studies of anti-inflammatory ac-tivity. Our data indicate that some compounds are able to control the NO and PGs bio-synthesis on inflammatory process through the in vitro inhibition of iNOS activity in J744.A1 macrophage cell line.
Keywords
Arachidonic acid, eicosanoids, prostaglandin, inflammation, cytotoxicity
Introduction
The arachidonic acid is a polyunsaturated fatty acid pre-sent in human body; it’s introduced trough the diet or it may arise from linoleic acid (essential fatty acid). In the cells, the arachidonic acid is linked to membrane phos-pholipids (phosphatidylcholine, phosphatidylinositole, phosphatidyletanolamine) and it is the precursor in the synthesis of eicosanoid; it’s metabolized to several groups of lipid mediators, including prostaglandins, leukotrienes, and lipoxins [1].
Cyclooxygenase (COX) catalyzes the conversion of ara-chidonic acid into prostaglandins (PGs), which play a sig-nificant role in health and disease in the gastrointestinal tract (GI) and in the renal, skeletal, and ocular systems. COX-1 is constitutively expressed and found in most normal tissues, whereas COX-2 can be expressed at low levels in normal tissues and is highly induced by pro-inflammatory mediators [2].
Also the leukotrienes comprise a family of products of the 5-lipoxigenase pathway of arachidonic acid metabolism. They are the chemical mediators of inflammatory reaction and anaphilaxis, and also have a powerful effect vasodi-lator and in the bronchoconstriction. Furthermore, arachi-donic acid is also used in anandamide’s biosynthe
sis [3].
The anandamide, or arachidonoiletanolammide (AEA), is part of a new class of lipid mediators, acting predomi-nantly olocrina and paracrine, collectively known as en-docannabinoids.
During inflammatory setting, both COX-2 and the induc-ible isoform of nitric oxide synthase (iNOS) are detected in a variety of cells, resulting in the production of large amounts of pro-inflammatory PGs and cytotoxic nitric oxide (NO) molecules. Increasing evidences suggest that intracellular concentrations of NO and PGs may be rele-vant in switching on/off inflammatory cells by modulating their own biosynthesis and NOS/COX enzymes [4].
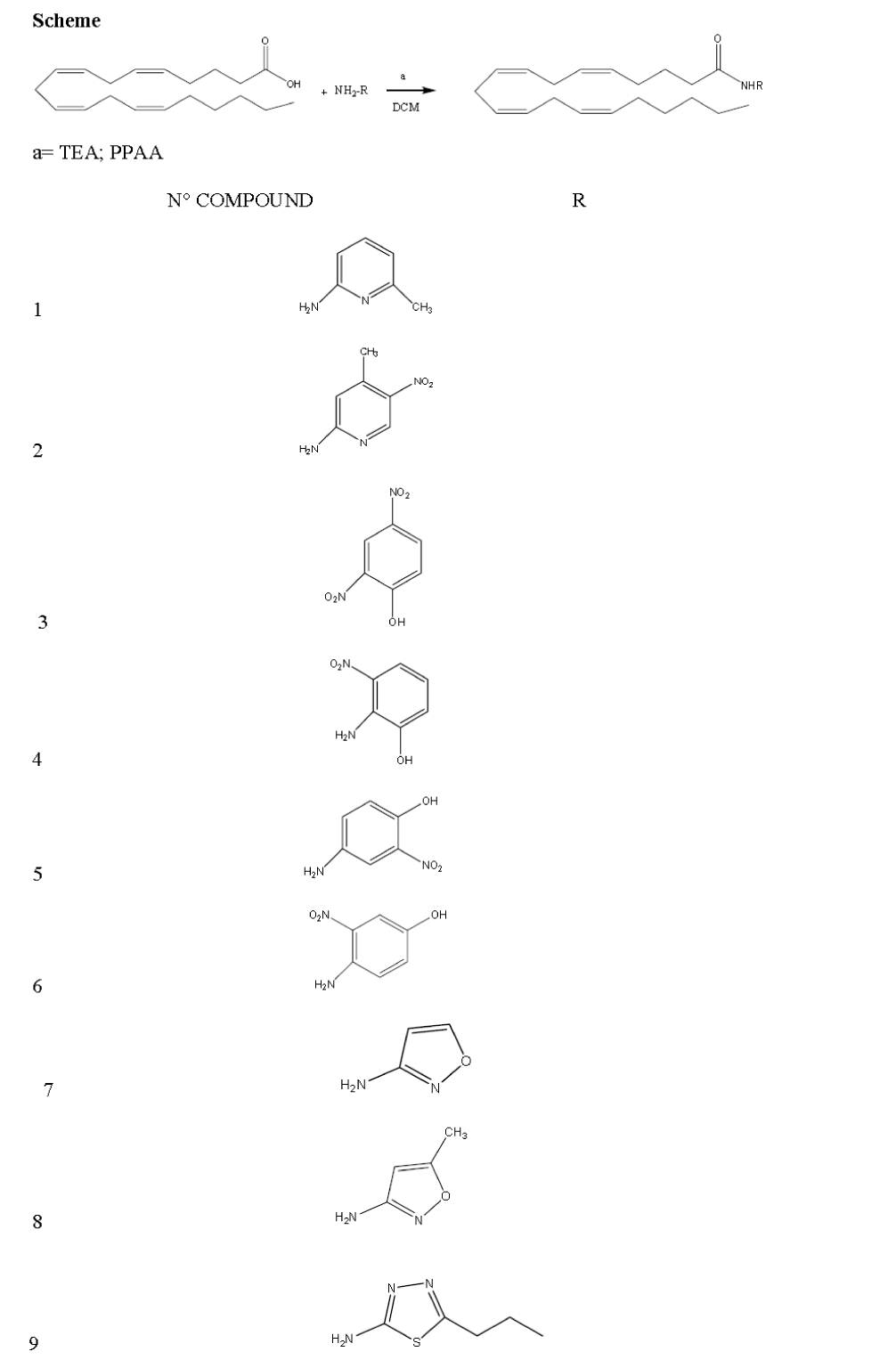
The goal of this work was to produce new compounds (1-9) obtained by adding a series of amines to the arachi-donic acid molecule and to evaluate their anti-inflammatory activity, preliminarily on NO production by LPS-induce macrophages.
Experimental section
Melting points were taken on a Gallenkamp melting point apparatus and are uncorrected. 1H NMR spectra were re-corded with a Bruker Avance 300 MHz spectrometer. Chromatographic separations were performed on silica gel column (Kiesalgel 40, 0040-0,063mm, Merck). Reac-tions and product mixtures were routinely monitored thin layer chromatography (TLC) on silica gel precoated F254 Merck plates.
General method of synthesis of products 1-9
Arachidonic acid (1eq) was added to a series of amines (1,5eq) in dichloromethane (2-3 ml) in the presence of TEA (4eq) and PPAA (2eq), at room temperature [5]. The solvent was removed under reduced pressure and the resi-due was purified by flash chromatography (silica gel) us-ing petroleum ether/ ethylacetate as eluent.
N-(6-methylpyridin-2-yl)icosa-5,8,11,14-tetraenamide (1).
Arachidonic acid (70mg, 0,23mmol, 1eq) is dissolved in dry DCM (2ml); to this solution are added: TEA (130μl, 0,92mmol, 4eq), PPAA (136μl, 0,46mmol, 2eq) and 2-amino-6-methylpyridine (38 mg, 0,35mmol, 1,5eq).
The reaction mixture is kept under reflux for about 7 hours, then the solvent is evaporated and the resulting residue was purified by silica gel column chromatography (eluents: petroleum ether/ethyl acetate 85:15) to get an oily compound with a yield of 50%.
1H NMR (CDCl3) :8.00 (s, 1H); 7.80 (d, 1H); 7.50 (t, 1H); 7.25 (d, 1H); 5.45-5.25 (m, 8H); 2.63-2.58 (m, 6H); 2.50 (s, 3H); 2.00 (m, 4H); 2.20 (t, 2H); 1.62 (m, 2H); 1.36-1.30 (m, 6H); 0.98 (t, 3H).
N-(4-methyl-5-nitropyridin-2-yl) icosa-5,8,11,14- tetra-enamide (2).
Arachidonic acid (70mg, 0.23mmol, 1eq) is dissolved in dry DCM (2ml); to this solution are added TEA (130μl, 0.92mmol, 4eq), PPAA (136μl, 0,46mmol, 2eq) and then is added 2-amino-4-methyl-5-nitropyridine (54mg, 0,35mmol, 1,5eq).
The reaction mixture is kept under reflux for about 5 hours, then the solvent is evaporeted and the resulting residue was purified by silica gel column (eluents: petroleum ether/ethyl acetate 85:15) to get an oily compound with a yeld of 60%.
1H NMR (CDCl3 ): 9.40 (s, 1H); 8.45 (s, 1H); 5.45-5.26 (m, 8H); 2.65 (m, 6H); 2.50 (s, 3H); 2.20 (t, 2H); 1.98 (m, 6H); 1.58 (m, 1H); 1.55-1.25 (m, 6H); 0.98 (t, 3H).
N-(2-hydroxy-5-nitrophenyl)icosa-5,8,11,14-tetraenamide (3).
Arachidonic acid (70mg,0.23mmol, 1eq) is dissolved in dry DCM (2ml); to this solution are added TEA (130μl, 0.92mmol, 4eq), PPAA (136μl, 0,46mmol, 2eq) and then is added 2-amino-4-methyl-5-nitropyridine (54mg, 0,35mmol, 1,5eq).
The reaction mixture is kept at room temperature for 5 hours; then the solvent is evaporeted and the resulting residue was purified by silica gel column (eluents: petroleum ether/ethyl acetate 9:1) to get an oily compound with a yeld of 55%.
1H NMR (CDCl3) : 8.28 (s, 1H); 7.90 (s, 1H); 7.86 (s, 1H); 7.00 (s, 1H); 5.45-5.35 (m, 8H); 4.90 (s, 1H); 2.60 (m, 6H); 2.25 (t, 2H); 2.00 (m, 4H); 1.72 (m, 2H); 1.38-1.28 (m, 6H); 0.99(t, 3H).
N-(2-hydroxy-5-nitrophenyl)icosa-5,8,11,14-tetraenamide (4).
Arachidonic acid (90mg, 0.30mmol, 1eq) is dissolved in dry DCM (3ml); to this solution are added TEA ( 165μl, 1.18mmol, 4eq), PPAA (177μl, 0.60mmol, 2eq) and then is added 2-amino-3-nitrophenol (70mg, 0.45mmol, 1.5eq).
The reaction mixture is stirred at room temperature, for 3 hours ; then the solvent is evaporeted and the resulting residue was purified by silica gel column chromatography (eluents: petroleum ether/ethyl acetate 7:3) to get an oily compaund with a yeld of 68%.
1H NMR (CDCl3): 8.80 (s, 1H); 7.80 (d, 1H); 7.20 (d, 1H); 7.18 (d, 1H); 5.48-5.37 (m, 8H); 4.20 (s, 1H); 2.62 (m, 6H); 2.25 (t, 2H); 2.00 (m, 4H); 1.72 (m, 2H); 1.30-1.27 (m, 6H); 0.98 (t, 3H).
N-(4-hydroxy-3-nitrophenyl)icosa-5,8,11,14-tetraenamide (5).
Arachidonic acid (90mg, 0.30mmol, 1eq) is dissolved in dry DCM (3ml); to this solution are added TEA (165μl, 1.18mmol, 4eq), PPAA (177μl, 0.60mmol, 2eq) and then is added 4-amino-2-nitrophenol (70mg, 0.45mmol, 1.5eq).
The reaction mixture is stirred at room temperature for 5 hours; then the solvent is evaporeted and the resulting residue was purified by silica gel column chromatography (eluents: petroleum ether/ethyl acetate 7:3) to get an oily compaund with a yeld of 65%.
1H NMR (CDCl3): 8.30 (s, 1H); 7.85 (t, 1H); 7.20 (s, 1H); 7.00 (d, 1H); 5.55-5.35 (m, 8H); 4.50 (s, 1H); 2.63 (m, 6H); 2.25 (t, 2H); 1.98 (m, 4H); 1.70 (m, 2H); 1.35-1.31 (m, 6H); 0.98 (t, 3H).
N-(4-hydroxy-2-nitrophenyl)icosa-5,8,11,14-tetraenamide (6).
Arachidonic acid (90mg, 0.30mmol, 1eq) is dissolved in dry DCM (3ml); to this solution are added TEA (165μl, 1.20mmol, 4eq), PPAA (177μl, 0.60mmol, 2eq) and then is added 4-amino-3-nitrophenol (55mg, 0.36mmol, 1.2eq).
The reaction mixture is stirred at room temperature for about 6 hours; then the solvent is evaporeted and the resulting residue was purified by silica gel column chromatography (eluents: petroleum ether/ethyl acetate 7:3) to get an oily compaund with a yeld of 60%.
1H NMR (CDCl3): 8.00 (s, 1H); 7.75 (d, 1H); 7.65 (s, 1H); 6.90 (s, 1H); 5.45-5.35 (m, 8H); 4.50 (s, 1H); 2.64-2.60 (m, 6H); 2.25 (t, 2H); 1.98 (m, 4H); 1.73 (m, 2H); 1.32-1.28 (m, 6H); 0.99 (t, 3H).
N-(isoxazol-3-yl)icosa-5,8,11,14-tetraaenamide (7).
Arachidonic acid (90mg, 0.30mmol, 1eq) is dissolved in dry DCM (3ml); to this solution are added TEA (160μl, 1.20mmol, 4eq), PPAA (177μl, 0.60mmol, 2eq) and then is added 5-amino-isoxazol (33μl, 0.45mmol, 1.5eq).
The reaction mixture is stirred at room temperature for about 6 hours; then the solvent is evaporeted and the resulting residue was purified by silica gel column chromatography (eluents: petroleum ether/ethyl acetate 7:3) to get an oily compaund with a yeld of 63%.
1H NMR (CDCl3 ): 8.50 (s, 1H); 8.00 (s, 1H); 7.40 (s, 1H); 5.48-5.39 (m, 8H); 2.68 (m, 6H); 2.19 (t, 2H); 1.98 (m, 4H); 1.58 (m, 2H); 1.30-1.27 (m, 6H); 1.00 (t, 3H).
N-(5-methylisoxazol-3-yl)icosa-5,8,11,14-tetraenamide (8).
Arachidonic acid (90mg, 0.30mmol, 1eq) is dissolved in dry DCM (3ml); to this solution are added TEA (165μl, 1.20mmol, 4eq), PPAA (177μl, 0.60mmol, 2eq) and then is added 3-amino-5-methylisoxazol (44.14mg, 0.45mmol, 1.5eq).
The reaction mixture is stirred at room temperature for about 6 hours; then the solvent is evaporeted and the resulting residue was purified by silica gel column chromatography (eluents: petroleum ether/ethyl acetate 7:3) to get an oily compaund with a yeld of 48%.
1H NMR (CDCl3): 8.50 (s, 1H); 7.40 (s, 1H); 5.46-5.37 (m, 8H); 2.62 (m,6H); 2.38 (m, 3H); 2.20 (t, 2H); 2.00 (m, 4H); 1.73 (m, 2H); 1.35-1.30 (m, 6H); 0.97 (t, 3H).
N-(5-propyl-1,3,4-thiadiazol-2-yl)icosa-5,8,11,14-tetraenamide (9).
Arachidonic acid (90mg, 0.30mmol, 1eq) is dissolved in dry DCM (3ml); to this solution are added TEA (165μl, 1.20mmol, 4eq), PPAA (177μl, 0.60mmol, 2eq) and then is added 2-amino-5-propylthiadiazol (58.13mg, 0.45 mmol, 1.5eq).
The reaction mixture is stirred at room temperature for about 6 hours; then the solvent is evaporeted and the resulting residue was purified by silica gel column chromatography (eluents: petroleum ether/ethyl acetate 7:3) to get an oily compaund with a yeld of 70%.
1H NMR (CDCl3): 8.00 (s, 1H); 5.45-5.30 (m, 8H); 2.63 (m, 6H); 2.58 (m, 2H); 2.20 (t, 2H); 1.95 (m, 4H); 1.68 (m, 2H); 1.66 (m,2H); 1.35-1.30 (m, 6H); 0.98 (t, 6H).
Toxicological and Pharmacological Methods
Materials
The murine macrophage cell line (J774A.1) the murine fibrosarcoma cells (WEHI-164) and human epithelial kidney cells (HEK-293) was obtained from American Tissue Culture Collection (ATCC). E. coli lipopolysac-charide (LPS) was obtained from Fluka (Milan, Italy). 3-(4,5-Dimethylthiazolyl-2-yl) 2,5-diphenyltetrazolium bromide (MTT), phosphate buffer solution (PBS), bovine serum albumin (BSA), were obtained from Sigma Chemi-cal Co. (Milan, Italy). Dulbecco's modified Eagle's me-dium (DMEM), penicillin/streptomycin, HEPES, gluta-mine, fetal calf serum (FCS), and horse serum were from Hy Clone (Euroclone-Cellbio, Pero, Milan, Italy).
Cells
J774.A1, murine monocyte/macrophage cells, were grown in adhesion on Petri dishes and maintained at 37 °C as previously described [6]. WEHI-164 and HEK-293 were maintained in adhesion on Petri dishes with DMEM supplemented with 10% heat-inactivated FCS, 25 mM HEPES, 100 u/mL penicillin, and 100 μg/mL streptomy-cin.
Cytotoxic Activity
J774A.1, WEHI-164 and HEK-293 (3.5 x 104 cells/well) were plated on 96-well microtiter plates and allowed to adhere at 37 °C in a 5 % CO2 atmosphere for 2 h. There-after, the medium was replaced with of fresh medium and serial dilution of each test compound was added and then the cells incubated for 72 h. Serial dilutions of 6-mercaptopurine, as reference drug, were also added to cells. Mitochondrial respiration, an indicator of cell vi-ability, was assessed by the mitochondrial-dependent re-duction of [3-(4,5-dimethylthiazol-2-yl)-2,5-phenyl-2H-tetrazolium bromide] (MTT) to formazan and cells viability was assessed accordingly to the method of Mosmann. Briefly 5 μL of MTT (5 mg/mL) were added and the cells were incubated for an additional 3 h. Thereafter, cells were lysed and the dark blue crystals solubilised with 100 μL of a solution containing 50% (v:v) N, N-dimethylformamide, 20 % (w:v) SDS with an adjusted pH of 4.5. The optical density (OD) of each well was meas-ured with a microplate spectrophotometer (Titertek Mul-tiskan MCC/340) equipped with a 620 nm filter. The vi-ability of each cell line in response to treatment with tested compounds and 6-mercaptopurine was calculated as: % dead cells=100-(OD treated/OD control)x 100 [7].
Analysis of Nitrite
J774A.1 (5.0 x 104 cells/well) were plated on 96-well mi-crotiter plates and allowed to adhere at 37°C in a 5% CO2 atmosphere for 2 h. Examined compounds (0.01-1 mM) was added 1h before and simultaneously to LPS (6x103 u/ml), used to induce inducible nitric oxide synthase (iNOS). Nitric oxide release (NO), evaluated as nitrite (NO2-) accumulation in the cell culture medium, were per-formed 24 h after LPS stimulation by Griess reagent [8]. The amount of nitrite in the samples was calculated from a sodium nitrite standard curve freshly prepared in culture medium. Results are expressed as percentages of inhibi-tion calculated versus cells treated with LPS alone.
Results and Conclusions
To establish the effects on cell viability of new com-pounds (1-9) obtained by adding a series of amines to the arachidonic acid in vitro, (0.01-1 mM), were each tested on J774.A1, WEHI-164, and HEK-293 cells using MTT test. Among tested compounds only 3, 5 and 6 didn’t show cytotoxic effect on cells (data not shown).
The involvement of NO on inflammatory process and its relation with PGs biosynthesis led us to investigate the in vitro activities of iNOS, evaluating NO biosynthesis, in J744.A1 macrophage cell line. NO release in the cellular medium of LPS-stimulated J774.A1 macrophages, incu-bated with new compounds which didn’t resulted cyto-toxic (0.01−1 mM) was evaluated 24 h after LPS (6 × 103 u/mL) challenge. Results were expressed as % of inhibi-tion calculated versus macrophages treated with LPS alone. The results of the present study indicate that com-pounds 5 and 6 added 1 h before and simultaneously with LPS, significantly inhibited at the concentrations 0.1 and (54.0%) 1m M (67.0%), NO release (P<0.001). Results on NO release also showed that compound 3 was the most active inhibiting at all tested concentrations, and in a re-lated- manner, NO release (P<0.001 vs LPS alone) (62.0% and 82,6%, respectively).
Therefore, our data indicate that compounds 3, 5 and 6 are able to control the NO and PGs biosynthe-sis on inflammatory process through the in vitro inhibi-tion of iNOS activity in J744.A1 macrophage cell line.
References
- Boyce JA. Eicosanoids in asthma, allergic inflamm-ation, and host defense, Cur Mol Med 2008; 8 (5): 335-349.
- Pecchi E, Dallaporta M, Jean A, Thirion S, Troadec JD. Prostaglandins and sickness behavior: Old story, new insights. Physiol. Behav. 2009 97, 279-292
- Wiley JL, Razdan RK, Martin BR. Evaluation of the role of the arachidonic acid cascade in anandamide’s in vivo effects in mice. Life Sci. 2006; 80(1): 24-35.
- Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of Prostaglandin Biosynthe-sis by Nitric Oxide and Nitric Oxide Donors, Pharma-col Rev 2005, 57: 217-252.
- Teall M, Harrison T, Moseley JD, Owens AP, Sadowski S, Cascieri MA. Bioorg Med Chem Lett 1996: 6: 1585-1588.
- Marzocco S, Piacente S, Pizza C, Oleszek W, Stochmal A, Pinto A, Sorrentino R, Autore G.. Inhibition of in-ducible nitric oxide synthase expression by yuccaol C from Yucca schidigera roezl. Life Sciences, 2004 75 (12), 1491-501.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assays. J Immunol Methods 1983; 65, 55-63.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS., Tannenbaum SR. Analysis of nitrate ni-trite and 15N-nitrite in biological fluids. Anal. Biochem 1983; 126 (1): 131-138.