ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2006) Volume 17, Issue 1
oxicity studies of the aqueous extract of Vernonia amygdalina
1Department of Pharmacology Lagos State Uni-versity College of Medicine, PMB 21266, Ikeja, Lagos, Nigeria
2Department of Morbid Anatomy Lagos State Uni-versity College of Medicine, PMB 21266, Ikeja, Lagos, Nigeria
3Department of Chemical Pathology Lagos State Uni-versity College of Medicine, PMB 21266, Ikeja, Lagos, Nigeria
4Department of Haematology Lagos State Uni-versity College of Medicine, PMB 21266, Ikeja, Lagos, Nigeria.
- *Corresponding Author:
- O.O. Amole
Departments of Pharmacology
Lagos State University College of Medicine PMB 21266
Ikeja, Lagos, Nigeria
Accepted December 11, 2005
The aqueous leaf extract, after six weeks administration to the animals showed no difference in the morphology of the heart, liver, kidney and intestinal tissues of the treated and un-treated animals. Body weights of both the treated and untreated animals rose progressively as the period increased. The plant extract produced no significant changes in packed cell volume (PCV), white blood cell (WBC) and platelet counts. This study suggests that the plant extract is non-hazardous, even when taken for some time.
Keywords
Toxicity,, Vernonia amygdalina, haematological parameters, biochemical parameters, histopathology
Introduction
Plants have served as valuable starting materials for drug development even in developed countries. Vernonia amygdalina (family, compositae) is a valuable shrub that is widespread in East and West Africa [1]. In Nigeria, it is commonly known as “bitter leaf” because the leaves and stem have a bitter taste when chewed. This is in addi-tion to its leaves being used as a popular vegetable for soups particularly among the Igbos of Southern Nigeria. Its use for fever, laxative, pile (haemorrhoids) and gastro-intestinal troubles have been reported [1,2]. Its anti-thrombic and anticoagulant properties have also been evaluated [3,4]. Oral administration of the aqueous leaf extract of the plant was found to relieve pain [5] and to lower body temperature [5].
In Nigeria recently there has been an increase in research into medicinal plants, with the view to providing alterna-tives to or supplementing imported drugs thereby preserv-ing our foreign exchange reserves. But toxic actions are a limitation to the potential usefulness of any agent and may even lead to deaths and Vernonia species are the sources of many local medicines. It is for this reason, that this study is primarily designed to determine the long-term effects which small and large doses of the extract might probably have on haematological and biochemical parameters and also on certain major organs of the body.
Materials and Methods
Preparation of extracts and phytochemical assay
Methods of preparation of extracts and phytochemical assay have been explained elsewhere [6].
Chronic toxicity test
30 male albino rats were administered with doses of the plant ranging from 87.53g kg-1 to 92.57g kg-1 to drink at will for 42 days. During the period of the study, weights of the animals were monitored weekly with their gross appearances. At the end of the 42nd day, the rats were killed by cervical dislocation. Post-mortem of each of the animals were performed. The heart, the liver, the kidneys, and the intestinal tissues were excised. The organs from the groups given the plant drug were compared with those of the controls. The specimens from the organs were fixed in 10% formol saline for histopa-thological studies.
Histological Methods
Small pieces of tissues taken at autopsy were fixed in 10% formol saline. The tissues were dehydrated through alcohol series, cleared in chloroform and finally embedded in paraffin wax (m.p. 56°C). They were sectioned on a rotatory microtome (R. Jung. Heideberg, Germany) and dried in an oven at 12 h at 56oc.°
Staining technique
Sections were deparaffinized by placing them in xylene for 2 min, taking them through Haematoxylin for 10 min and they were finally washed in tap water. Counterstain-ing was carried out with 1% aqueous Eosin, and the specimen were dehydrated in alcohol, cleared in xylene before mounting.
Sections from all tissues were prepared and examined in this way for any evidence of histopathological changes.
Haematological parameters
Blood samples were collected through heart puncture of each diethyl ether anaesthetized rat into different sample bottles. The blood samples were analyzed for packed cell volume (PCV), white blood cell (WBC) and platelet counts.
Biochemical parameters
The effects of the extract on certain biochemical parame-ters were compared with those of the control. Serum from the blood of each rat was collected into Lithium heparin bottles and mixed with necessary reagents, Plasma assays for tests on the function of liver viz serum alanine amino transferase (ALT) and aspartate amino transferase (AST) were done within 24 hours of collection using an autoanalyzer (Smart Lab Analyzer).
Statistical analysis
Results are reported as mean ± SEM. Statistical analysis was carried out using Student’s t-test. Significance was considered at values of P<0.0.5.
Results and Discussion
This study is very important in order to determine the cumulative effect of V. amygdalina in body tissues, since no scientific evidence has ever been adduced of the effect of prolonged use of the decoction. Slides of the excised and prepared tissue sections namely, the heart, liver, kidney and intestinal tissues were observed microscopically and showed no morphological abnormalities as compared with the controls.
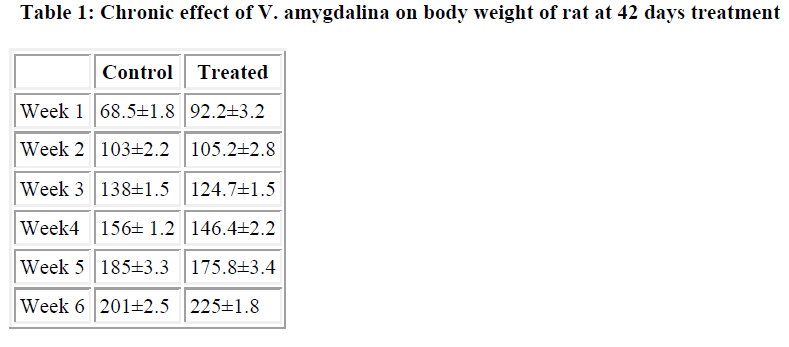
There were no significant changes in the heart, liver, kidney and intestinal tissues. The animals in both the treated and untreated groups showed no physical changes in their appearances. Body weight of both the treated and untreated animals rose progressively as the duration increased (Table 1).
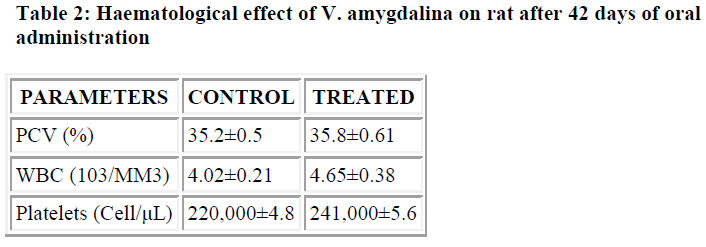
Chronic treatment with V. amygdalina produced no sig-nificant changes in PCV, WBC and platelet counts (Table 2). Some of the results obtained as regards biochemical parameters suggest the need for further assessment and evaluation. In conclusion, this study suggests that the aqueous extract of this plant may be safe, even when taken for some time.
Mean weight of animals (g)
References
- Ainslie, J.R. List of plants used in native medicine in Nigeria. Imperial Forestry Institute, Oxford 1973, 42
- Oliver, B. Medicinal plants in Nigeria. Nigeria College of Science and Technology. 1960, 50-51
- Awe, S.O., Olajide, O.A. and Makinde, J.M. Effect of Allium sativum and Vernonia amygdalina on thrombosis in mice. Phytotherapy Research 1998 12, 57- 58
- Igboechi, A.C. and Anuforo, D.C. Anticoagulant activities of extracts of Eupatorium odoratum and Vernonia amygdalina. Nig. J. Pharm. 1984, 20, 313- 320
- Tekobo, A.M., Onabanjo, A.O., Amole, O.O. and Emeka P.M. Analgesic and antipyretic effects of the aqueous extract of Vernonia amygdalina. West Afri. J. Pharm. 2002 16, 68-74
- Celia, M. and Wilkinson, W. Liver function – A review. Aust Vet. J. 1989 49, 163– 169.