ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2020) Volume 31, Issue 3
Phenolic acids from Coffea arabica L. suppress intestinal uptake of glucose and cholesterol.
Acharaporn Duangjai1*, Pattadon Pontip2, Sutidaporn Sumhem2, Wutthichai Kaweekul2, Maleeruk Utsintong2, Atcharaporn Ontawong1, Kanittaporn Trisat3, Surasak SaoKaew4-9
1Unit of Excellence in Research and Product Development of Coffee, Division of Physiology, School of Medical Sciences, University of Phayao, Phayao, Thailand 5600
2School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand 56000
3Department of Pharmacy Practice and Center of Excellence for Innovation in Chemistry, Pharmacological Research Unit, Pharmaceutical Sciences, Naresuan University, Phitsanulok, Thailand
4Center of Health Outcomes Research and Therapeutic Safety (Cohorts), School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
5Unit of Excellence on Clinical Outcomes Research and IntegratioN (UNICORN), School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
6Unit of Excellence on Herbal Medicine, School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
7Division of Pharmacy Practice, Department of Pharmaceutical Care, School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
8Biofunctional Molecule Exploratory Research Group, Biomedicine Research Advancement Centre, School of Pharmacy, Monash University Malaysia, Bandar Sunway, Selangor DarulEhsan, Malaysia
9Novel Bacteria and Drug Discovery Research Group, Microbiome and Bioresource Research Strength, Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Bandar Sunway, Selangor DarulEhsan, Malaysia
- Corresponding Author:
- Acharaporn Duangjai
Division of Physiology
School of Medical Sciences
University of Phayao
Phayao
Thailand
Accepted date: May 28, 2020
The This study assessed the hypoglycaemic and hypocholesterolaemic effects of coffee fruit extracts and their active compounds involved in cholesterol and glucose uptake, as well as the inhibitory activity of α-glucosidase and α-amylase, the physiochemical changes of cholesterol micelles, and the uptake of glucose and cholesterol in Caco-2 cells. The results revealed that coffee green dry (CGD) coffee yellow dry (CYD), and coffee red bean dry (CRBD) extracts inhibited α-amylase activity by approximately 49% whereas caffeine and caffeic acid, showed high potency to inhibit α-amylase activity by 94.49% and 93.35%, respectively. In addition, coffee red pulps dry (CRPD) and citric acid inhibited αglucosidase by 29% and 46%, respectively. The extracts (1 mg/mL) and their active compounds (200 µM) inhibited glucose uptake in Caco-2 cells. All coffee fruit extracts inhibited cholesterol uptake in Caco-2 cells by approximately 40%, and they decreased the micellar solubility of cholesterol, except for coffee red fresh (CRF) and coffee red dry (CRD). Moreover, CYD strongly increased the size of cholesterol micelles. These findings demonstrate the potential effect of coffee extracts on anti-diabetic activity and their cholesterol-lowering activity according to the inhibitory activity of α-glucosidase and α-amylase and the uptake of glucose and cholesterol in Caco-2 cells.
Keywords
Alpha-glucosidase, Alpha-amylase, Coffee fruit, Diabetes, Caco-2 cells.
Introduction
Cancer Dyslipidaemia in patients with type 2 diabetes (T2DM) is associated with an increased risk of cardiovascular disease (CVD), which is the prevalent cause of comorbidity in diabetic patients [1]. Diabetes mellitus (DM) is a metabolic disorder characterised by the presence of hyperglycaemia [2], while dyslipidaemia refers to conditions where there are elevated cholesterol and triglyceride levels or decreased high-density lipoprotein (HDL) cholesterol levels [3]. One of the therapeutic approaches diabetes, postprandial hyperglycaemia, is to reduce the absorption of glucose from the intestine by inhibiting carbohydrate-hydrolysing enzymes and suppressing the absorption of glucose, whereas the management of dyslipidaemia involves the inhibition of cholesterol synthesis and absorption. However, drugs come with side effects and a high cost of treatment. The use of bioactive compounds and extracts from natural resources as an alternative treatment has minimal adverse effects.
Coffee belongs to Coffea genus and the Rubiaceae family. There are many nutrients and bioactive compounds in coffee, including dietary fibre, carbohydrates, chlorogenic acid, caffeine, citric acid, quinic acid, ferulic acid, melanoidins, and trigonelline [4,5]. In addition, it has been suggested that coffee by-products (coffee husk, parchment, and silverskin) enriched in phytochemicals and antioxidant dietary fibre are healthpromoting food ingredients [6]. Coffee and its bioactive ingredients have been associated with a lower risk of several chronic diseases, including CVD; antibacterial, anti-diabetic, neuroprotection, and anti-cancer activities [7], age-related cognitive decline; and Alzheimer ’ s disease [8]. More recently, it was shown that coffee fruit extracts have antiadipogenic and lipolytic properties, which may protect against obesity [4]. However, the mechanism underlying the cholesterol- and glucose-lowering effects of coffee fruit extracts remains unclear. The potential mechanisms involved in these effects were evaluated in this research. The proposed mechanisms for the hypoglycaemic and hypocholesterolaemic effects of coffee fruit extracts may involve suppressed cholesterol and glucose uptake. In this study, differentiated Caco-2 cells were used to clarify the effects of coffee fruit extracts and their active compounds on intestinal glucose and cholesterol uptake, as well as to examine their effect on α- amylase and α-glucosidase inhibitory actions and physiochemical changes in cholesterol micelles.
Materials and Methods
Chemicals
Folin-Ciocalteu reagent; sodium carbonate; gallic acid; 1,1- diphenyl-2-picrylhydrazyl (DPPH); 2,2 ’ -azino-bis(3- ethylbenzthiazoline-6-sulfonic acid) (ABTS); phosphatidylcholine; cholesterol; sodium taurocholate; 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT); dimethyl sulfoxide (DMSO); ethanol; Dulbecco's modified Eagle's medium (DMEM)/F12; foetal bovine serum; penicillin – streptomycin; trypsin – EDTA; [1,2(n)-3H]cholesterol; ezetimibe; NaOH; Triton-X 100; acarbose; α-amylase; starch; sodium phosphate buffer; dinitrosalicylic acid (DNS); α-glucosidase; p-nitrophenyl-α-Dglucopyranoside (pNPG); potassium phosphate buffer; and 2- NBDG were used.
Preparation of coffee extracts
Green, yellow, and red coffee fruits were obtained from Chao- Thai-Pukao Factory, Chiang Mai, Thailand. Aqueous coffee fruit extracts were prepared according to a previous report [4]. Eight extracts, coffee red fresh (CRF), coffee red dry (CRD), coffee green fresh (CGF), coffee green dry (CGD), coffee yellow fresh (CYF), coffee yellow dry (CYD), coffee red pulp dry (CRPD), and coffee red bean dry (CRBD), were stored at –20°C for further use.
Proximate analyses
All parameters of the proximate analyses, including ash, moisture, crude protein, fat, fibre, and nitrogen-free extract (NFE), were estimated as described by the Association of Official Analytical Chemists (AOAC, 2010) [9].
Determination of total phenolic compounds
The total phenolic content of coffee fruit extracts was determined by the Folin-Ciocalteu method [10]. Gallic acid was used as the standard phenolic compound. The extract (1 mg/mL) was incubated with Folin-Ciocalteu’s reagent and sodium carbonate (7.5%) at room temperature for 30 min absorbance was measured at 750 nm. The total phenolic content was calculated as the gallic acid equivalent (GAE) in milligrams per gram of dry material.
Determination of antioxidant activity
Determination of DPPH radicals scavenging activity: The radical scavenging activity was evaluated using DPPH as previously reported [11]. Various concentrations of the extracts were incubated with methanolic DPPH solution. After 30 min incubation in the dark and at room temperature, absorbance was measured at 510 nm. The capability to scavenge the DPPH radical was expressed as 50% of the radical scavenging activity (IC50).
Determination of ABTS radical scavenging activity: The radical scavenging activity was determined by ABTS [12]. The ABTS cation radical reagent was prepared by mixing ABTS solution with potassium persulfate solution (1:1). The mixture was stored at room temperature in the dark for 12-16 h and diluted with water before use. Various concentrations of the extracts (100 μL) were incubated with ABTS reagent (100 μL) at room temperature for 30 min and then absorbance was measured at 734 nm. The ABTS cation radical capacity was represented as the IC50 value.
In vitro intestinal cholesterol absorption
Determination of cholesterol micelle solubility: The method used to determine cholesterol micelle solubility was slightly modified from that of Kirana et al. [13]. The final concentrations of the mixed micelle solutions contained 0.6 mMphosphatidylcholine, 1 mM cholesterol, and 1 mM sodium taurocholate. The micelle solutions were incubated with coffee fruit extracts (1 mg/mL) at 37°C for 3 h. After incubation, the mixed solution was filtered through a 0.22 μm filter, and the cholesterol micelle solubility was determined using a cholesterol by cholesterol assay kit.
Determination of cholesterol micelle size: The micelle size assay was modified from Kirana et al. [13]. A cholesterol micellar solution was prepared according to a previous study [14,15]. Coffee fruit extracts (1 mg/mL) were incubated with the micelle solution at 37°C for 3 h. After incubation, the particle size of the mixed solution was measured using a particle size analyser.
Determination of cell viability: Caco-2 cells were provided from American Type Culture Collection (ATCC) and cultured in DMEM/F12 supplemented with 10% foetal bovine serum and 1% penicillin–streptomycin and maintained at 37°C in an atmosphere of 5% CO2 and 95% humidified air. The cells were suspended using trypsin–EDTA and plated in either 96-well plates or culture flasks.
Caco-2 cell viability was evaluated using the MTT assay. Cells were seeded at a density of 10,000 cells per well in 96-well plates and maintained for 24 h. After incubation with coffee fruit extracts (1 mg/mL) and their active compounds (200 μM) for 22 h, 10 μL of MTT (5 mg/mL) was added and the solution was further incubated for 2 h. The culture medium was removed and solubilised formazan dye with 200 μL of dimethyl sulfoxide: ethanol (1:1) and then measured at 595 nm. The data are expressed as the percentage of viable cells.
Determination of cholesterol uptake: Micellar cholesterol solution was prepared for the uptake assay, as describe previously [14,15]. The final concentration of the [3H]-labelled micellar solution was as follows: 1 Ci/mL[1,2(n)-3H]cholesterol, 1 M cholesterol, 50 M phosphatidylcholine, and 2 mM sodium taurocholate.
After differentiation of Caco-2 cells, coffee fruit extracts (200 µg/mL) or ezetimibe (40 µg/mL) were pre-treated for 1 h, and the [3H]-labelled micellar solution was added to the culture dishes, which were left undisturbed for 3 h. After incubation, the cells were rinsed two times with ice-cold PBS and lysed in 0.2 N NaOH/0.1% Triton-X 100. The radioactivity in the cellular lysates was determined and then normalised by protein contents.
In vitro anti-diabetic activity
Determination of α-amylase inhibitor activity: The α- amylase inhibitory activity was determined according to a previous study [16,17], with slight modifications. In brief, mixing solutions of 50 μL of coffee fruit extracts (5 mg/mL), their active compounds (200 μM), or acarbose (200 μM) were pre-incubated with α-amylase (100 μL) at 37°C for 10 min, and then 100 μL of starch (2 mg/mL) in 0.25 M sodium phosphate buffer (pH 7.0) was added. After further incubation at 37°C for 20 min, the reaction was terminated by adding 1% DNS (100 μL). The mixing solution was further incubated at 90°C for 5 min and cooled to room temperature. The absorbance was then measured at 540 nm. The α-amylase inhibitory activity was calculated as the percent inhibition. Acarbose was used as a positive control.
Determination of α-glucosidase inhibitor activity: The α- glucosidase inhibitory activity was examined using the method by Quan et al. [18] with slight modifications. Briefly, coffee fruit extracts (5 mg/mL), their active compounds (5 mM), or acarbose (2 mM) were pre-incubated with α-glucosidase solution in 0.1 M potassium phosphate buffer (pH 7.0). After 15 min of incubation, 100 μL of 400 μM pNPG in potassium phosphate buffer was added and incubated for 40 min. The reaction mixture was stopped by adding 0.1 M Na2CO3 and absorbance was measured at 405 nm. The results are expressed as the percentage of α-glucosidase inhibitory activity.
Determination of glucose uptake: Caco-2 cells were seeded in 96-well plates at a density of 1 × 104 cells per well and cultured for 14-21 days. Differentiated cells were placed in serum-free media overnight prior to the uptake studies. The cells were pre-equilibrated for 1 h with glucose-free media containing extracts, and then 2 mM fluorescent glucose 2- NBDG was added to initiate uptake. The reaction was terminated after 4 h by washing with ice-cold PBS three times. The 2-NBDG uptake by cells was detected at an excitation of 485 nm and an emission of 535 nm. The results are expressed as the percentage of glucose uptake relative to the control.
Statistical analyses
The experiments were performed with at least three independent replicates. All data are expressed as the mean ± standard error of the mean (S.E.M.) Statistical analysis was performed using the paired two-tailed Student’s t-test, with p<0.05 considered to indicate statistical significance.
Results
Nutritional values of coffee fruit extracts
The proximate compositions in the coffee fruit extracts are summarized in Table 1. Proximate compositions of extracts expressed in 100 g dry weight basis, a moisture content of 5.37–10.97, ash content of 9.59–18.52, fat content of 0.60– 2.99, protein content of 7.70–17.63, fibre content of 0.06– 1.36, NFE content of 53.20–64.98, and calorie content of 296.09–353.66. The results revealed that coffee fruit extracts have nutritional value with important biological activities.
| % Dry samples | % Moisture | % Ash | % Fat | % Protein | % Fiber | % NFE | Calories (Kcal) | |
|---|---|---|---|---|---|---|---|---|
| CRF | 92.13 ± 0.01 | 7.87 ± 0.01 | 14.42 ± 0.04 | 1.72 ± 0.09 | 12.79 ± 0.05 | 0.23 ± 0.01 | 62.97 ± 0.14 | 318.49 ± 0.39 |
| CRD | 89.59 ± 0.04 | 10.41 ± 0.04 | 15.67 ± 0.10 | 1.29 ± 0.06 | 12.00 ± 0.08 | 0.29 ± 0.01 | 60.33 ± 0.15 | 300.94 ± 0.36 |
| CGF | 93.17 ± 0.04 | 6.83 ± 0.04 | 17.11 ± 0.06 | 0.83 ± 0.04 | 16.81 ± 0.10 | 0.06 ± 0.01 | 58.36 ± 0.08 | 308.17 ± 0.60 |
| CGD | 90.91 ± 0.04 | 9.09 ± 0.04 | 18.52 ± 0.15 | 2.77 ± 0.03 | 16.34 ± 0.15 | 0.08 ± 0.01 | 53.20 ± 0.02 | 303.09 ± 0.71 |
| CYF | 90.84 ± 0.03 | 9.16 ± 0.03 | 11.73 ± 0.07 | 1.55 ± 0.07 | 13.53 ± 0.03 | 0.29 ± 0.01 | 63.75 ± 0.02 | 323.05 ± 0.81 |
| CYD | 90.78 ± 0.04 | 9.22 ± 0.04 | 13.40 ± 0.10 | 1.69 ± 0.02 | 13.42 ± 0.03 | 0.10 ± 0.01 | 62.17 ± 0.17 | 317.59 ± 0.53 |
| CRBD | 94.63 ± 0.03 | 5.37 ± 0.03 | 9.59 ± 0.07 | 2.99 ± 0.06 | 17.63 ± 0.15 | 0.36 ± 0.01 | 64.06 ± 0.09 | 353.66 ± 0.40 |
| CRPD | 89.03 ± 0.05 | 10.97 ± 0.05 | 15.65 ± 0.08 | 0.60 ± 0.00 | 7.70 ± 0.05 | 0.10 ± 0.01 | 64.98 ± 0.05 | 296.09 ± 0.43 |
Table 1: Nutritional value per 100 g of coffee fruits extract.
Total phenolic content and antioxidant activity
The total phenolic content and antioxidant activity of the coffee fruit extracts are shown in Table 2. The total phenolic content in the extracts was found to be in the range of 36.67– 56.82 GAE mg/g extract. The antioxidant activity with DPPH and ABTS is represented as IC50 values. The antioxidant activity was found to be in the range of 1.277–34.3 μg/mL and 21.27–31.51 μg/mL for DPPH and ABTS, respectively.
| Antioxidant activity | Polyphenol Gallic acid equivalence (GAE) mg/g extract |
||
|---|---|---|---|
| DPPH IC50 µg/ml |
ABTS IC50 µg/ml |
||
| CRF | 2.069 ± 1.08 | 21.99 ± 1.05 | 48.63 ± 0.63 |
| CRD | 1.968 ± 1.16 | 26.64 ± 1.06 | 49.30 ± 0.31 |
| CGF | 1.971 ± 1.10 | 23.99 ± 1.04 | 52.50 ± 1.36 |
| CGD | 1.965 ± 1.12 | 24.84 ± 1.04 | 52.68 ± 2.12 |
| CYF | 2.41 ± 1.13 | 24.61 ± 1.05 | 47.70 ± 0.37 |
| CYD | 1.947 ± 1.08 | 31.51 ± 1.06 | 48.92 ± 0.71 |
| CRBD | 1.277 ± 1.09 | 21.27 ± 1.06 | 56.82 ± 1.18 |
| CRPD | 34.3 ± 1.37 | 28.9 ± 1.08 | 36.67 ± 0.22 |
Table 2: Total polyphenol content and antioxidant activity of coffee fruit extracts.
Effect on Caco-2 cell viability
The cytotoxicity of coffee fruit extracts was examined by the MTT assy. All coffee fruit extracts (1 mg/mL) and their active compounds (200 μM) showed no significant cytotoxic effects on Caco-2 cells, as shown in Figure 1. Therefore, we used these concentrations of the extracts and their active compounds for further experiments on Caco-2 cells.
Effect on in vitro intestinal cholesterol absorption
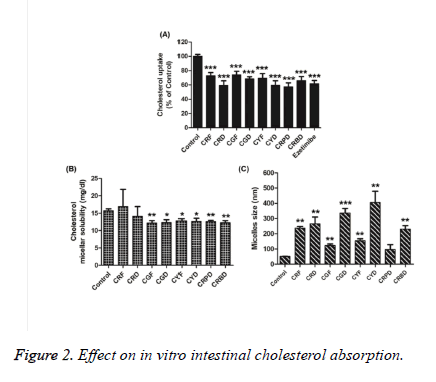
To investigate whether these coffee fruit extracts might regulate intestinal cholesterol absorption, the effect of the coffee fruit extracts on cholesterol uptake in differentiated Caco-2 cells, the cholesterol micellar solubility, and the cholesterol micelle size were examined. All coffee fruit extracts (200 μg/mL) showed cholesterol uptake inhibition in Caco-2 cells in the range of 26–40% of the control (Figure 2A). In addition, the micellar solubility of cholesterol decreased significantly in the presence of all coffee fruit extracts, except CRF and CRD, when compared to the control, as shown in Figure 2B. As represented in Figure 2C, all extracts increased micelle size of cholesterol, except CRPD, when compared to the control. In particular, CYD revealed an increased cholesterol micelle particle size of approximately 406 nm, while size of control showed 51 nm. These results revealed that coffee fruit extracts have potential cholesterol lowering ability.
Effect on in vitro anti-diabetic activity
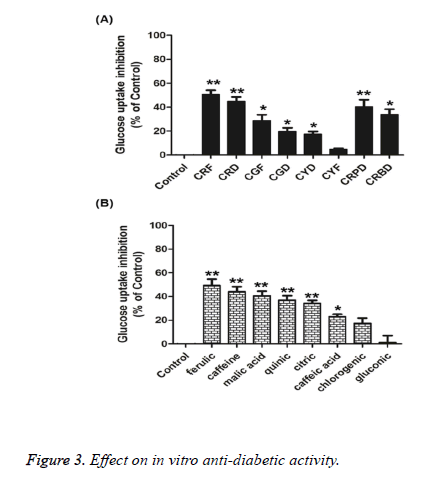
Here, we investigated whether these coffee fruit extracts and their active compounds might regulate intestinal glucose absorption. All coffee fruit extract (1 mg/mL) treatments showed significant glucose uptake inhibition of approximately 17–50%, as shown in Figure 3A, except CYD, and they were positively correlated with their active compounds (200 μM), ferulic acid, caffeine, malic acid, quinic acid, citric acid, caffeic acid, and gluconic acid, except for chlorogenic acid, as shown in Figure 3B.
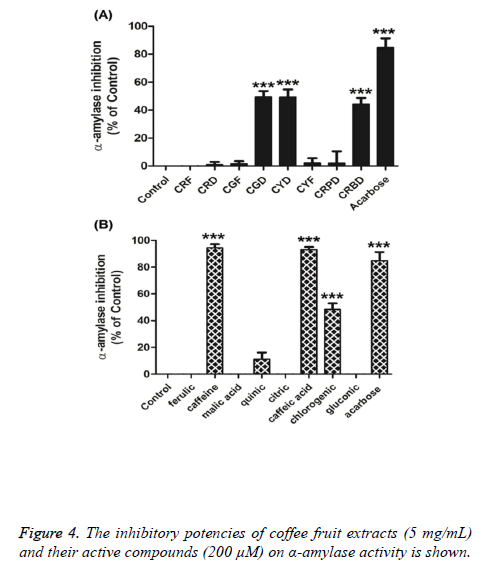
The inhibitory potencies of coffee fruit extracts (5 mg/mL) and their active compounds (200 μM) on α-amylase activity are summarised in Figure 4. The results revealed that CGD, CYD, and CRBD extracts significantly inhibited α-amylase activity by 49.30, 49.32, and 44.24%, respectively. Interestingly, caffeine and caffeic acid showed high potency to inhibit α- amylase activity by 94.49% and 93.35%, respectively, while acarbose (200 μM) inhibited α-amylase activity by 84%. These data indicate that caffeine and caffeic acids are as potent at inhibiting α-amylase activity as acarbose.
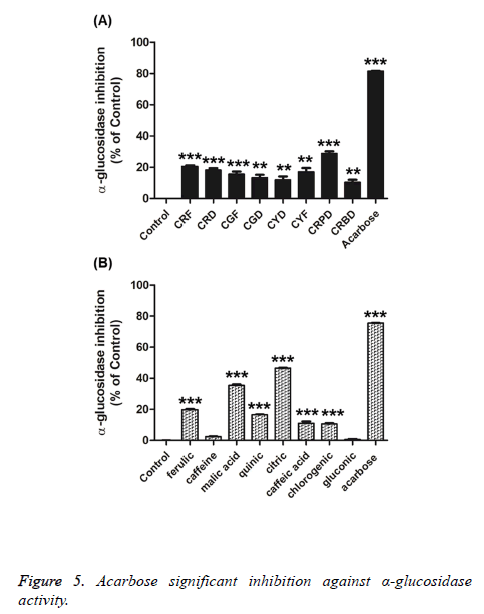
Furthermore, the in vitro α-glucosidase inhibition demonstrated that both the coffee fruit extracts (5 mg/mL) and their active compounds (5 mM) (except for caffeine and gluconic acid) had α-glucosidase inhibitory activity. The percentage of α- glucosidase inhibition of the extracts and their active compounds are expressed in the range of 11.95–28.85% and 10.62–46.57%, respectively. Acarbose (2 mM), a positive control, showed significant inhibition of 75.53% against α- glucosidase activity, as shown in Figure 5. These results show that coffee fruit extracts and their active compounds possess antidiabetic activity.
Discussion
The risk of CVD can increase through many risk factors, such as increased triglycerides, high LDL cholesterol, low HDL cholesterol, and elevated blood glucose [1]. Evidence shows that the gastrointestinal tract plays a significant role in postprandial plasma glucose regulation [19], Dietary carbohydrates are digested by pancreatic α-amylase to disaccharides, sucrose, and lactose. These metabolites are degraded to monosaccharides by α-glucosidase and further absorbed into the intestine. Thus, the inhibition of α-amylase and α-glucosidase function is one therapeutic approach to treat diabetic patients [20].
Glucose is then transported across the brush border membrane (BBM) in the form of glucose via Na+–glucose co-transporter type 1 (SGLT1) [21]. Glucose molecules are absorbed through the basolateral membrane (BLM) by glucose transporter type 2 (GLUT2) [22]. A previous study reported that an increase in SGLT-1 expression leads to elevation of the intestinal glucose absorption rate and the development of obesity [23]. Upregulation of SGLT-1 expression induces intestinal hypertrophy, resulting in postprandial hyperglycaemia, insulin resistance, and hyperinsulinaemia in Otsuka Long-Evans Tokushima fatty rats [24]. In addition, the expression of intestinal glucose transporters increases in diabetic patients compared to that of healthy controls [22]. Therefore, several compounds that can interfere with intestinal glucose absorption have received attention.
Coffee fruit extract has several nutritional ingredients, fibres, and polyphenols. The major polyphenols found in coffee fruit extract have been identified, including caffeoylquinic acid, chlorogenic acid, and caffeic acid [4]. This study revealed that coffee fruit extracts, including CRF, CRD, and CRPD, strongly inhibit glucose transport (p<0.01). These extracts interfered with only α-glucosidase activity and not with α-amylase activity. Therefore, these extracts may have multiple concomitant mechanisms to inhibit glucose transport. Several natural compounds also inhibit glucose absorption through down-regulation of glucose transporter expression, such as black bean protein hydrolysates, strawberry, and apple extract [25,26].
This study also showed that CGD, CYD, and CRBD interrupt both enzyme functions; α-glucosidase and α-amylase and also glucose absorption. Similarly, caffeic acid, which is an active pure compound of coffee fruit, exhibited the anti-diabetic effect by inhibiting α-glucosidase, α-amylase, and glucose absorption. However, CYF ’ s effects involve neither interfering with enzymatic action nor reducing glucose transport. These data imply that various colours of coffee fruit extract have different effects on glucose delivery. In addition, this study clearly demonstrated that coffee fruit extract enriched with polyphenols has high radical scavenging effects. These results are consistent with a previous study showing that caffeic acid inhibited lipid peroxidation by 68.2% compared to that of the control. Caffeic acid is also scavenged the ABTS+, DPPH scavenging and superoxide anion radicals [27]. Moreover, CGA, a major component of coffee fruit extract, increased the superoxide anion scavenging activity and inhibited iron-induced lipid peroxidation, resulting in brain oxidative stress suppression in a rat model [28].
Intestinal cholesterol absorption is a critical step for cholesterol homeostasis in the body. It has been revealed that dietary lipids in the form of cholesterol micelles are absorbed into enterocytes by the cholesterol transporter Niemann Pick C1 Like 1 (NPC1L1) [29]. Additionally, a study indicated that incomplete micellar complex formation influences human intestinal cholesterol absorption [30]. Thus, compounds that interfere with micellar incorporation are also needed. This study demonstrated that all coffee fruit extracts rich in polyphenols also showed a lipid lowering effect. Furthermore, the related molecular mechanisms of coffee fruit extracts on intestinal cholesterol transport were clarified. This study demonstrates the interference of cholesterol micellar formation by CGF, CGD, CYF, CYD, and CRBD, involving two mechanisms, including elevation of cholesterol micelle particle size and reduction of cholesterol micelle solubility. Similarly, our previous study indicated that coffee pulp extract inhibits cholesterol absorption via an increase in the micelle size of cholesterol and a reduction in cholesterol solubility [31].
Conclusion
In conclusion, the data obtained in this study indicate that coffee fruit extracts interfere with glucose and cholesterol absorption at the intestinal epithelial level, suggesting that they might be useful in the reduction of the risk of diabetes, dyslipidaemia, and CVD.
Acknowledgement
This research was supported by the University of Phayao, Phayao, Thailand under grant [grant numbers RD62065]; and Unit of excellence [grant numbers UoE62007 and UoE63004], University of Phayao, Thailand; a talent mobility program 2018, office of the higher education commission, and national science technology and innovation policy office, Thailand; the NSTDA Chair Professor Grant (the Fourth Grant) of the Crown Property Bureau Foundation and the National Science and Technology Development Agency to Professor Dr.VatcharinRukachaisirikul.
References
- Battisti WP, Palmisano J, Keane WF. Dyslipidemia in patients with type 2 diabetes. Relationships between lipids, kidney disease and cardiovascular disease. CCLM 2003; 41: 1174-1181.
- Baynes HW. Classification, pathophysiology, diagnosis and management of diabetes mellitus. J Diabetes Metab 2015; 6: 1-9.
- Klop B, Elte JWF, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrient 2013; 5: 1218-1240.
- Duangjai A, Nuengchamnong N, Suphrom N, Trisat K, Limpeanchob N, Saokaew S. Potential of Coffee Fruit Extract and Quinic Acid on Adipogenesis and Lipolysis in 3T3-L1 Adipocytes. Kobe J Med Sci 2018; 64: E84-E92.
- Nuhu AA. Bioactive micronutrients in coffee: recent analytical approaches for characterization and quantification. ISRN Nutr 2014; 384230.
- Iriondo-DeHond A, Garcia NA, Fernandez-Gomez B, Guisantes-Batan E, Escobar FV, Blanch GP, Andres IS, Sanchez-Fortun S, Castillo MD. Validation of coffee by-products as novel food ingredients. Innov Food SciEmergTechnol 2019; 51: 194-204.
- Hu GL, Wang X, Zhang L, Qiu MH. The Sources and Mechanisms of Bioactive Ingredients in Coffee. Food Funct 2019; 10: 3113-3126
- Carman AJ, Dacks PA, Lane RF, Shineman DW, Fillit HM. Current evidence for the use of coffee and caffeine to prevent age-related cognitive decline and Alzheimer’s disease. J Nutr Health Aging 2014; 18: 383-392.
- AOAC Minerals: In Official Methods of analysis. Washington, DC: Association of Official Analytical Chemists 2010.
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. AJEV 1965; 16: 144-158.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958; 181: 1199-1200.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free RadicBiol Med 1999; 26: 1231-1237.
- Kirana C, Rogers PF, Bennett LE, Abeywardena MY, Patten GS. Naturally derived micelles for rapid in vitro screening of potential cholesterol-lowering bioactives. J Agric Food Chem 2005; 53: 4623-4627.
- Duangjai A, Limpeanchob N, Trisat K, Amornlerdpison D. Spirogyra neglecta inhibits the absorption and synthesis of cholesterol in vitro. IMR 2016; 5: 301-308.
- Yamanashi Y, Takada T, Suzuki H. Niemann-Pick C1-like 1 overexpression facilitates ezetimibe-sensitive cholesterol and β-sitosterol uptake in CaCo-2 cells. J PharmacolExpTher 2007; 320: 559-564.
- Caraway WT. A stable starch substrate for the determination of amylase in serum and other body fluids. Am J ClinPathol 1959; 32: 97-99.
- Kazeem M, Adamson J, Ogunwande I. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of MorindalucidaBenth leaf. Biomed Res Int 2013: 527570.
- Quan NV, Xuan TD, Tran H-D, Thuy NTD, Trang LT, Huong CT, Andriana Y, Tuyen PT. Antioxidant, α-Amylase and α-Glucosidase Inhibitory Activities and Potential Constituents of Canariumtramdenum Bark. Molecules 2019; 24: 605.
- Holst JJ, Gribble F, Horowitz M, Rayner CK. Roles of the Gut in Glucose Homeostasis. Diabetes Care 2016; 39: 884-892.
- Ouassou H, Zahidi T, Bouknana S, Bouhrim M, Mekhfi H, Ziyyat A, Legssyer A, Aziz M, Bnouham M. Inhibition of alpha-Glucosidase, Intestinal Glucose Absorption, and Antidiabetic Properties by Carallumaeuropaea. Evid Based Complement Alternat Med 2018: 9589472.
- Shirazi-Beechey SP. Molecular biology of intestinal glucose transport. Nutr Res Rev 1995; 8: 27-41.
- Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J PhysiolGastrointest Liver Physiol 2002; 282: G241-G248.
- Osswald C, Baumgarten K, Stumpel F, Gorboulev V, Akimjanova M, Knobeloch KP, Horak I, Kluge R, Joost HG, Koepsell H. Mice without the regulator gene Rsc1A1 exhibit increased Na+-D-glucose cotransport in small intestine and develop obesity. Mol Cell Biol 2005; 25: 78-87.
- Fujita Y, Kojima H, Hidaka H, Fujimiya M, Kashiwagi A, Kikkawa R. Increased intestinal glucose absorption and postprandial hyperglycaemia at the early step of glucose intolerance in Otsuka Long-Evans Tokushima Fatty rats. Diabetologia 1998; 41: 1459-1466.
- Manzano S, Williamson G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol Nutr Food Res 2010; 54: 1773-1780.
- Mojica L, Luna-Vital DA, Gonzalez de Mejia E. Black bean peptides inhibit glucose uptake in Caco-2 adenocarcinoma cells by blocking the expression and translocation pathway of glucose transporters. Toxicol Rep 2018; 5: 552-560.
- Gulcin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006; 217: 213-220.
- Lee K, Lee JS, Jang HJ, Kim SM, Chang MS, Park SH, Kim KS, Bae J, Park JW, Lee B, Choi HY, Jeong CH, Bu Y. Chlorogenic acid ameliorates brain damage and edema by inhibiting matrix metalloproteinase-2 and 9 in a rat model of focal cerebral ischemia. Eur J Pharmacol 2012; 689: 89-95.
- Brown JM, Yu L. Protein mediators of sterol transport across intestinal brush border membrane. Subcell Biochem 2010; 51: 337-380.
- Woollett LA, Wang Y, Buckley DD, Yao L, Chin S, Granholm N, Jones PJ, Setchell KD, Tso P, Heubi JE. Micellar solubilisation of cholesterol is essential for absorption in humans. Gut 2006; 55: 197-204.
- Ontawong A, Duangjai A, Muanprasat C, Pasachan T, Pongchaidecha A, Amornlerdpison D, Srimaroeng C. Lipid-lowering effects of Coffea arabica pulp aqueous extract in Caco-2 cells and hypercholesterolemic rats. Phytomedicine 2019; 52: 187-197.