ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2016) Volume 27, Issue 2
Physicochemical properties of MTA, CEM, hydroxyapatite and nano hydroxyapatite-chitosan dental cements.
| Mehrnaz Hosseinzade, Reza Karimi Soflou, Azam Valian, Hanieh Nojehdehian* Department of Dental Materials, School of Dentistry, Shahid Beheshti University of Medical Sciences, Iran |
| Corresponding Author: Hanieh Nojehdehian, Department of Dental Materials School of Dentistry Shahid Beheshti University of Medical Sciences Iran |
| Accepted: January 30, 2016 |
Objective: Dental cements used as fillers in endodontics are in direct contact with the alveolar bone and bone regeneration by these cements is an important factor for their clinical success. The ability to form new hard tissue and its integration with the surrounding tissues are determined by the process of apatite deposits formation on bioactive ceramics. This study aims to evaluate the bioactivity of Mineral trioxide aggregates, Calcium enriched mixture, hydroxyapatite and nano hydroxyapatite-chitosan cements.
Materials and methods: Samples disks of these cements immersed in Simulated Body Fluid (SBF) solution were evaluated on days 1, 7 and 14 through measuring pH as well as examining the cement surface morphology and calcium to phosphorus ratio via Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Analysis (EDXA) respectively and Fourier Transform Infrared Spectroscopy (FTIR) analysis of apatite deposited chemical groups.
Results: The nano hydroxyapatite-chitosan and hydroxyapatite cements have neutral pH values, and CEM and MTA cements are “slightly basic” and “very basic” respectively. The SEM results revealed that apatite crystals would in time form on the surface of the cements. The EDXA results showed that calcium and phosphorus increased in all cements as time passed.
Conclusion: All the tested cements demonstrated bioactivity. The CEM and the nano hydroxyapatitechitosan cements demonstrated better bioactive features in terms of pH values.
Keywords |
||||||||
| Bioactivity, MTA, CEM, HA, Nano HA-Chitosan, Endodontics cement | ||||||||
Introduction |
||||||||
| As filling agents, endodontic cements are in direct contact with the alveolar bone and have clinical applications in the following fields: apical dentine replacement, pulp capping, pulpotomy, creation perforation and barriers in teeth with open apices, repair of root perforation and resorptive defects, as well as orthograde or retrograde root canal fillings, root-end filling materials , root perforation repair, pulp capping, root canal sealers, and dentin hypersensitivity [1]. | ||||||||
| The apatite phase formation and bone regeneration potential are important factors in clinical success of dental cements [2]. Osteoconductive and osteoinductive materials in cements determine the rate of forming new bone tissue of these cements and the percentage of bone regenerating [3]. | ||||||||
| Ceramics are divided into two types: neutral ceramics and bioactive ceramics. In interacting with their biological environment, neutral ceramic materials cause the creation of a fibrous layer which binds the implant to the bone in a non-rigid way, whereas the bioactive ceramics generate hard tissues which ultimately lead to the formation of a firm bond between the implant and the bone [4]. | ||||||||
| Osteogenesis is closely correlated with material bioactivity. Therefore, increasing bioactivity in dental and bone cements is of particular significance. The Bioactivity Index is an indicator of the rate of bone tissue generation and the level of connection between biomaterials and natural bone tissue. The shorter the bonding time the greater the rate of ion exchange leading to the formation of calcium phosphate on the surface and crystallization of the material. These reactions ultimately lead to the formation of stoichiometric hydroxyapatite. In order to assess the bioactivity of materials, Kokubo suggests evaluating their in vitro interaction with simulated body fluid, SBF [5,6]. | ||||||||
| The process of hydroxyapatite formation on materials is shown through morphological examination of the apatite crystals formed and comparison of the resulting calcium to phosphorus with that of the hydroxyapatite used. Bioactivity is related to the size and type of the crystalline and amorphous phases existing in the structure and the ion exchange between the cement material and the environment. In this study, the bioactivity of the two commercial cements, namely, the calcium silicate cement, Mineral Trioxide Aggregate (MTA Angelus; 1990), and the calcium-rich cement, Calcium- Enriched Mixture (CEM; 2006), are compared with that of the calcium phosphate hydroxyapatite cement and the nano hydroxyapatite-chitosan based composite cement. MTA is a bioactive and biocompatible hydrophilic calcium-silicate based cement which releases calcium in the presence of human body physiological fluids, leading to bone differentiation and stimulating mineralization of dental pulp stem cells [7,8]. CEM also possesses good bioactivity and biocompatibility, and contains several antibacterial calcium compounds including calcium hydroxide, calcium sulphate, calcium silicates, calcium oxide, and calcium phosphate [9,10]. Both these cements are successfully employed in clinical applications [11,12]. Many studies have been conducted on the properties of MTA and CEM. In one study, it was shown that CEM had better properties than nano hydroxyapatite in direct pulp capping [13]. | ||||||||
| Calcium phosphate cements, such as hydroxyapatite are considered as an appropriate choice for endodontic applications. Due to higher solubility and precipitation of calcium and phosphate ions and structural similarity between hydroxyapatite of setting reaction to dental hydroxyapatite [14,15]. Due to the Nano hydroxyapatite, in the composition of Nano hydroxyapatite- chitosan cements, considerable effect on cellular behaviour are expected, in a biological environment [16,17]. Also, chitosan as a member of GAG proteoglycans category, a main Extra Cellular Matrix component (with antibacterial and osteoconductive properties), can significantly affect cell behaviour during formation of hard tissue [18-20].In the conducted studies, it was shown that nano hydroxyapatite had a positive effect on biocompatibility, proliferation, and differentiation of mesenchymal stem cells [21]. Also, the researchers used ZnO as an additive in the nano hydroxyapatite cement [22]. | ||||||||
| The common characteristic of these cements in the biological environment was the calcium-dependent ADPs causing them to interact with CO2 dissolved in tissue fluids resulting in the production of carbonate apatite crystals. These effects played an important role in the biocompatibility of these materials and their influence on reactions between proteins and cells [7]. | ||||||||
| Although, in the previous studies, bioactivity of the mentioned cements was examined individually the collective bioactivity thereof has not been studied. This research was studied with the aim of bioactivity evaluation of commercial dental cements and research cements for improving properties and performance of cements in future. | ||||||||
Materials and Methods |
||||||||
| The MTA (Angelus, Brazil) and CEM (Bionique Dent, Tehran, Iran) cements were obtained commercially, and the compounds for hydroxyapatite and composite nano hydroxyapatitechitosan cements were prepared according to the following table1. Chitosan (Medium Molecular weight; 448877) was obtained from Sigma-Aldrich; USA. Nano HA and Tetra Calcium Phosphate (TTCP) were procured from Materials and Energy Research Center (MERC), Alborz, Iran. Calcium hydrogen phosphate dehydrate (DCPD); Sodium dihydrogen phosphate dehydrate (NaH2PO4); di-Sodium hydrogen phosphate (Na2HPO4); Acetic Acid (CH3COOH); and Citric Acid were purchased from Merck Millipore, Germany. | ||||||||
Sample preparation |
||||||||
| The disk-shaped samples of the CEM cement and MTA cement, as well as hydroxyapatite, and nano hydroxyapatitechitosan cements were prepared according to their powder/ liquid ratio (Table 1). Upon initial setting and removing the disks from the molds, the samples were treated in the incubator for 24 hours at 37ºC. | ||||||||
pH variation |
||||||||
| pH variation of the environment is highly significant in the reconstruction and functioning of induction osteo/dentino/ cementogenic cements [23]. Daily readings of pH variations of the samples submerged in SBF inside the incubator were obtained from the pH meter apparatus (Jenway 3310, UK) for seven days [6]. The pH variation tests were repeated 3 times for further accuracy. | ||||||||
Cement bioactivity |
||||||||
| To study the bioactivity of the tested cements, we placed the samples inside the SBF prepared through the Kokubo method [6]. After being placed inside the incubator for 24 hours, the prepared disks were transferred to 24 well plates each containing 3 cc of SBF and left there for 14 days. On the 1st, 7th, and 14th days, the samples were removed, rinsed briefly with twice distilled water, and dried under the chemical hood. | ||||||||
SEM and EDXA |
||||||||
| An SEM was used to study the morphology and calcium precipitation on the surface of the cement disks. Upon coating the samples with gold, the Vega/Tescan apparatus was used to take SEM photographs from the samples. The quantitative EDXA was implemented for bioactivity study, determining calcium to phosphorus ratio, and identifying the elements in the cement structure. | ||||||||
FTIR analysis |
||||||||
| The FTIR analysis was conducted in the wave number range of 4000-400 cm-1on the SBF-submerged samples using the apparatus (Thermo-Nicholet, Nexus 870, USA). The analysis was conducted on days 1, 7, and 14 to examine the formation of chemical groups in the cement structures. | ||||||||
Statistical analysis |
||||||||
| Using the SPSS (version 19.0) relevant mean and standard deviation values as well as percentages were calculated. Due to the low sample size, the Kruskal-Wallis (p-value) test was conducted to study the significant difference between quantitative variables. A significance level of less than 0.05 was considered. | ||||||||
Results |
||||||||
pH variations |
||||||||
| pH variations were due to the setting of cements. As observed in Table 2, the hydroxyapatite and the composite nano hydroxyapatite cements neutralized their environment (pH=7), the CEM made their surrounding environment basic, and the MTA made their surrounding environment highly basic. | ||||||||
SEM and EDXA results |
||||||||
| The SEM images showed that small flower-shaped and needlelike crystals had formed in the MTA and CEM samples submerged in SBF for one day (Figure 1). In the nano hydroxyapatite sample, nano cluster crystals were formed, and in the hydroxyapatite cement, small cement particles were observed together with certain larger crystals. | ||||||||
| The SEM images obtained for the samples submerged in SBF for 7 days showed that ceramic crystals had formed and developed in all the samples. In the MTA cement, larger flower-shaped crystals had appeared besides other crystal growths. In CEM cement few small needle-like crystals were seen beside other crystal growths. In the hydroxyapatite cement, small needle-like crystals had covered the sample surfaces. In the nano hydroxyapatite cement, needle-like crystals were observed on the polymer matrix, which appeared to be calcium phosphate crystals. | ||||||||
| After 14 days of submersion in SBF, calcium phosphate precipitation and small crystals thereof were observed in the MTA cement sample. The SEM images obtained for the CEM samples did not exhibit any particular morphological change (as compared to the previous state). In the hydroxyapatite samples, a sharper needle-like structure was observed. The nano clusters had disappeared from the nano cements and crystalline particles were observed instead. The EDXA results indicated that calcium and phosphorus had increased with time in all the tested groups as shown in Table 3 and 4. According to these results, the Ca, P, and Si elements found on the MTA and CEM had increased far more than other groups during days 7 and 14. This can be attributed to formation of a hydrated calcium silicate gel layer on the surface of these cements [7]. In the nano hydroxyapatite cement, very slight Ca and P variations were observed with time, probably due to Ca solubility and absence of silica as a result of polymerization in the environment. The hydroxyapatite sample demonstrated a slight increase in Ca as time passed. | ||||||||
FTIR results |
||||||||
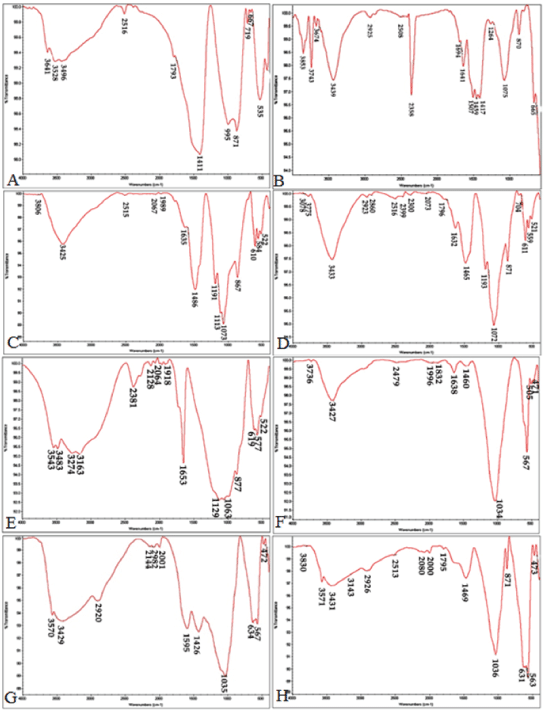
| The FTIR analysis was conducted on the cements upon setting to study the chemical groups formed in the samples before and after immersing them in the SBF (Figure 2). The FTIR analysis before and after the samples were submerged in the SBF showed peaks for different functional groups to occur at the following spectral loci: | ||||||||
| • Silicate: 522-535 cm-1 | ||||||||
| • Calcium silicate: 871, 876 and 955 cm-1 | ||||||||
| • Phosphate: 564, 567, and 1035 cm-1 | ||||||||
| • Carbonate: 871 cm-1 | ||||||||
| • Calcite (a subgroup of the calcium carbonate group) : 1411 cm-1 | ||||||||
| • Calcite aragonite: 1063 and 1073 cm-1 | ||||||||
| • Calcium carbonate: 1420-1460 cm-1 | ||||||||
| • OH: 3528, 3543, and 3425 cm-1 | ||||||||
| • Chitosan: 1595, 1426, 2920, and 3429 cm-1 | ||||||||
| With increasing submergence time in SBF, the flexural and tensile vibrations in the P-O bonds deepened. Deeper phosphate peaks and their increased number were indicative of formation of hydroxyapatite on the sample surface. Due to apatite precipitation, the peaks increased at 1030 and 560-600 wave lengths. Also, the increase in the following wave numbers can indicate apatite carbonate precipitation: 1460, 600, 870, 960, 1025, 1410, and 560. The peak observed at 1075 wave length in the MTA sample (submerged in the SBF) points to the continued presence of chitosan peaks. Moreover, the FTIR results showed that the silicate functional group (polymerized silicate tetrahedral) was no longer present at 535 nm in the MTA and CEM samples 14 days after the start of the test. | ||||||||
Discussion |
||||||||
| Protecting the pulp and keeping the tooth alive during restorative treatment as well as pulp and dentin regeneration using alternative materials are among the ideal targets in dentistry. These methods would prolong the life and efficiency of the tooth, through eliminating the need for root canal therapy. For this reason, it is essential to use a material which can provide the required coating for the direct and indirect pulp cap and, at the same time, can effectively regenerate more dentin. Bioactivity is closely related to the bone conduction capability of materials. In fact, the bioactivity index is related to the formation of hydroxyapatite on the surface of the utilized material. The purpose of the present study is to examine the bioactivity characteristic of the conventional dental cements MTA and CEM, and their comparison with the newly developed research cements, namely, hydroxyapetite and composite nano hydroxyapatite chitosan. Immersing these cements in the SBF (which contains ions similar to those found in blood plasma) causes the hydroxyapatite to precipitate onto the surface of the material in the form of needle-like crystals which support formation of new tissues, ultimately leading to tissue integrity. Calcium carbonate precipitation occurs more often in the form of calcite rather than apatite; a fact that can be observed in the FTIR results. | ||||||||
| The MTA Angelus was composed of tricalcium silicate, dicalcium silicate, tricalcium aluminate and bismuth oxide [12]. The SiO2 in the MTA cement acts as a suitable bed for apatite precipitation and nucleation [24]. The EDXA results showed the presence of Mg, Al, Si as well as C, P, O, and Ca in the MTA cement and Ca, P, O, and C in other groups. These results were in good agreement with those obtained by Camilleri et al. and Asgari et al. [9,25]. | ||||||||
| Calcium phosphate cements are bioactive ceramics with good biocompatibility properties as well as suitable bone conduction, bone integration, and ductility characteristics. However, these cements do not possess optimal mechanical features. For this reason, biopolymers such as chitosan can be used as matrix and binder in these cements to provide good mechanical properties as well as biocompatibility and lack of solubility [24]. It should be noted that the positively charged chitosan leads to a beneficial interaction with adjacent tissue cells, thus increasing osteogenesis and angiogenesis activities in the composite cement. The calcium carbonate and calcium oxide components are used to improve mechanical properties as well as increasing the setting rate of these materials [26]. | ||||||||
| Variations in pH affect the physicochemical and biological properties of cements. Solubility of the existing cement ions in the biological environment determines pH variations in the environment. These variations affect the interaction between the cement and the environment. An acidic environment causes inflammation of surrounding tissues [22]. A high pH in the environment due to high calcium ion solubility would lead to increased interaction with phosphate and antibacterial properties as well as formation of hydroxyapatite with hard surrounding tissues [23]. However, this process would also accelerate the precipitation of unstable spherical particles which quickly undergo phase transmutation and are transformed into planar structures, thus postponing the formation of bone-like apatite [22]. | ||||||||
| The pH ranges were obtained as follows for the studied cements: | ||||||||
| • HA: 7.92 ± 0.14 (neutral) | ||||||||
| • Composite nano hydroxyapatite- chitosan: 7.04 ± 0.11 (neutral) | ||||||||
| • CEM cement: 10.28 ± 0.74 (slightly basic) | ||||||||
| • MTA: 12.05 ± 0.11 (totally basic due to release of Ca (OH)2). | ||||||||
| CEM cement exhibited the least pH variation and produced a pH in the neutral range. This would reduce inflammatory response in the surrounding tissues and influence the formation of bone-like apatite. CEM also has bactericidal effects against endodontic pathogens. This property, in addition to the suitable sealing ability of CEM, may explain its successful use in treatment of external root resorption. These results were in good agreement with those obtained by Shahi [23]. | ||||||||
| The calcium phosphate precipitation process as well as morphology of the generated crystals determines the bioactivity level of a particular cement and the way the cement functions in the vicinity of hard tissue. Smaller particles would cause greater hydration and strength. Also, through generating surface roughness, smaller particles might absorb more protein, leading in turn to further absorption of proteins that exist in the biological system and those secreted by the cells to induce greater adhesion and cell proliferation [9]. | ||||||||
| Immersing the samples in SBF in time led to increased Ca and P as well as formation of needle-like hydroxyapatite crystals which also was observed in the HA cement. The dimensions of these needle-like crystals in the CEM cement were greater on day 14 as compared with the previous days. The size of hydroxyapatite cement particles was larger than the other cements. Whereas in the nano hydroxyapatite cement the nanoparticles formed clusters which in turn were transformed in 14 days into rod-shaped particles. Because MTA releases Ca and phosphate ions which are critical factors in bone metabolism and contributes to the formation of HA in synthetic biologic fluids, its favorable biologic properties are thus not surprising. The weight ratio of calcium ions to phosphate ions (the Ca/P ratio) determines the degree of bioactivity in a material. This weight ratio was obtained as 2.15% for the hydroxyapatite cement. Higher Ca/P weight ratios indicate calcium precipitation on the material’s surface and lower ratios indicate an incomplete setting reaction and formation of stoichiometric hydroxyapatite. The Ca/P ratios in the MTA cement samples during the first 24 hours, after 7 days, and after 14 days were obtained as 3.84, 8.33, and 2.74. This can indicate Ca precipitation and ion exchange in the silicate groups during the first 7 days and formation of calcium phosphate after 14 days. The Ca/P ratios obtained from conducting the EDXA results on the nHA cements for the first, the seventh, and the fourteenth days, indicate that Ca had precipitated on the cement which leads to good bioactivity, biocompatibility and osteoconductivity properties. EDXA results for the HA cement also indicates that, Ca precipitation on the HA cement was less than nHA cement. | ||||||||
| The FTIR results showed, the formation of the carbonate apatite phase had initiated and increased apatite adsorption capability and Formation of the phosphate group at 1075 in this spectrum was clearly detected upon the lapse of 14 days. The spectrum obtained for the CEM cement revealed the following functional groups: | ||||||||
| • Silicate functional group (polymerized tetrahedral silicate) at 522 | ||||||||
| • Apatite carbonate phosphate: at 564 and 610 | ||||||||
| • Hydrated calcium aluminate sulfate (Ettringite): at 1113 | ||||||||
| • Calcium sulfate: at 1191 | ||||||||
| • Carbonate: at 1438 and 1635 | ||||||||
| • Calcite: at 867. | ||||||||
| Peak absorption intensity increased upon SBF submergence for 14 days. In the hydroxyapatite cement, the HA peaks grew sharper upon SBF submergence, in both hydroxyapatite cement and nano hydroxyapatite cement the calcium carbonate or calcium oxide peaks (1400-1500) were reduced, indicating the purity of the apatite formed in these cements. | ||||||||
| The unchanged nano hydroxyapatite cement peaks indicate that chitosan does not undergo any chemical reaction in the formation of the this composite cement [27]. However, upon 14 days immersion the chitosan NH2 peak intensity in the nano hydroxyapatite cement decreased, and the phosphate group peak intensity increased and the carbonate group peak became more distinctive at 871, indicating that calcium phosphate and calcium carbonate had formed on the cement surface which can promote differentiation process of stem cells. | ||||||||
| According to the results obtained, immersing the MTA and CEM cements in SBF led to the formation of a silica gel on the surface, In return chitosan polymer has a same role in nano hydroxyapatite cement composite, the bioresorbable process in hydroxyapatite cement, controls the stoichiometric hydroxyapatite formation. | ||||||||
Conclusion |
||||||||
| The ability to regenerate dentin is an important characteristic to be considered in choosing materials for direct and indirect pulp capping. Bioactivity behavior of cements for this purpose are closely related to the bone conduction capability of the material. The results in the present study show that bioactivity of the nano hydroxyapatite cement was greater than that of other cements, suggesting that the particle size of the filler influences this behavior. | ||||||||
Tables at a glance |
||||||||
|
||||||||
Figures at a glance |
||||||||
|
||||||||
References |
||||||||
|