ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 15
Plasminogen activator inhibitor-1 gene is associated with radioactive lung injury in lung cancer patients
Department of Oncology, Heilongjiang Province Nongken General Hospital, Harbin, PR China
- *Corresponding Author:
- Huixia Liu
Department of Oncology
Heilongjiang Province Nongken General Hospital
Harbin, PR China
Accepted date: April 19, 2017
Although the application of radiotherapy techniques reduced normal lung tissue of exposure for patients with non-small cell lung cancer, Radioactive Pneumonia (RP) and Pulmonary Fibrosis (RF) are still frequently occurred in the processes of lung cancer radiotherapy. Plasminogen Activator Inhibitor-1 (PAI-1) plays a crucial role in the process of lung injury during RP in lung cancer patients. However, the relationships between PAI-1 and lung injury have not well understood, yet. In this study, we investigated the role of PAI-1 in the progression of RP and PF in patients with lung cancer, who have undergone radiotherapy. A total of 126 lung cancer patients and 62 healthy volunteers were recruited to analyse the changes of PAI-1 serum levels. We showed that PAI-1 serum levels were down-regulated in patients with lung cancer compared to healthy volunteers. Outcomes demonstrated that PAI-1 was up-regulated in lung cancer patients after radiotherapy. Patients with RP presented higher serum levels of PAI-1 were positively correlated with degree of RP and PF for patients who undergone radiotherapy. We also showed that serum levels of PAI-1 were down-regulated after recovery of radioactive lung injury in lung cancer patients. Outcomes indicated that PAI-1 gene expression levels were up-regulated in lung fibroblasts in patients with radioactive lung injury compared to healthy controls. In conclusion, these findings indicate that serum levels of PAI-1 were associated with radioactive lung injury-induced PR and PF in lung cancer patients, who undergone radiotherapy.
Keywords
PAI-1, Radiotherapy, Radioactive lung injury, RP, PF.
Introduction
Lung cancer is one of the most common human tumors in the world that causes the most common cancer-related mortality in the world [1,2]. Many reports have reported that lung tumor cells growth migration contributes to tumor metastasis, development and reoccurrence [3,4]. In recent years, more and more therapeutic schedules were applied for the treatment of human lung cancer and outcomes presented certain anti-cancer effects for clinical patients [5,6]. A review has showed the role of radiotherapy and targeted agents for medical treatment of advanced non-small cell lung cancer in elderly patients, which confirmed the anti-cancer efficacy of radiotherapy [7].
Lung injury induced by radiotherapy is frequently occurred in lung cancer patients after received radiotherapy [8,9]. Cella et al. have predicted the risk of developing asymptomatic or symptomatic pulmonary damage in hodgkin's lymphoma patients treated with sequential chemo-radiotherapy [10]. Evidences have showed that radiotherapy often leads to Radioactive Pneumonia (RP) and Pulmonary Fibrosis (RF) for patients with lung cancer [11,12]. Previous study has indicated that modified laryngotracheal separation for intractable aspiration pneumonia is potentially reversible in patients with lung carcinoma treated by radiotherapy [13]. In addition, a meta-analysis has demonstrated the association between TGFbeta1 polymorphisms and radiation pneumonia in lung cancer patients treated with definitive radiotherapy [14]. Furthermore, a biostatistician's perspective study has explored the application of competing risk model in oncology practice in head and neck cancer patients undergoing radical radiotherapy [15]. However, the biomarkers in the patients with radioactive lung injury remains poorly understand.
Plasminogen Activator Inhibitor-1 (PAI-1) plays a crucial role in the process of lung injury during RP in lung cancer patients [16]. Osterholzer et al. have indicated that PAI-1 can promote the accumulation of exudate macrophages and worsens pulmonary fibrosis following type II alveolar epithelial cell injury [17]. In this study, we investigated serum levels of PAI-1 in lung cancer patients prior and post treatment with radiotherapy. We reported that serum levels of PAI-1 are positively correlated with the degree of RP and RF. Here, we also analysed PAI-1 gene expression in lung fibroblasts in patients with radioactive lung injury compared to healthy controls.
Materials and Methods
Study design, subjects and sampling
A total of 126 lung cancer patients and 62 healthy volunteers were recruited in this retrospective study. The age of lung cancer patients was 24.7-62.8 y. Subjects include 72 male and 52 female cancer patients. Healthy volunteers included 30 male and 32 female. Inclusion criteria for individuals with lung cancer were diagnosed by CT and pathological sections [18]. The Institutional The study protocol was approved by the Central Ethics Committee (Ethics Committee of Heilongjiang Province Nongken General Hospital). All patients were required to write informed consent with signature.
ELISA
Blood samples were obtained from patients on day 200 after received radiotherapy. Serum levels of PAI-1 (MBS01873, Thermo Fisher Scientific) were analysed in healthy volunteers and patients with radioactive lung injury using ELISA kit according to the manufacturer’s instrument. The serum concentration levels of PAI-1 were measured by an enzyme micro-plate reader at 570 nm.
Real-time quantitative PCR (RT-qPCR) assay
Total RNA in lung cells was extracted using RNAzol, and DNase RNase-free was adopted to digest total RNA at 37°C for 15 min, and then RNeasy kit to purify RNA to adjust its concentration to 1 μg/μl. The 2 μg RNA was used as the template to synthetize cDNA by reacting with reverse transcriptase at 37°C for 120 min, at 99°C for 4 min, and at 4°C for 3 min respectively. Followed by, reverse transcriptionpolymerase chain reaction method was adopted to amplify the gene expression of PAI-1 (Forward 5’- GGCACAATCCAACAGAGACAA-3’, Reverse 5’- GGCTTCTCATCCCACTCTCAAG-3’) to determine the transcription level of mRNA, and β-actin was used as the housekeeping genes of internal control group. Eventually, agarose electrophoresis with 1% ethidium bromide was adopted to check PCR amplified products. Relative mRNA expression changes were calculated by 2-ΔΔCt. The results are expressed as the n-fold way compared to control.
Western blotting
Lung cells were collected and lysed in RIPA buffer (M-PER reagent for the cells and T-PER reagent for the tissues, Thermo Scientific) followed homogenized at 4°C for 10 min. A total of 20 μg protein extracts was electrophoresed on 12.5% polyacrylamide gradient gels and then transferred to nitrocellulose membranes. The membranes were incubated in blocking buffer (5% milk) prior to incubation with primary antibodies at 4°C overnight. The primary rabbit anti-human antibodies used in the immunoblotting assays were: PAI-1 (1: 500, ab125687, Abcam) and β-actin (1: 500, ab8226, Abcam). Horseradish peroxidase-conjugated anti-rabbit IgG (Bio-Rad, Hercules, CA, USA) were used at a 1: 5000 dilution and detected using a Western Blotting Luminol Reagent.
Regression analysis
The serum levels of PAI-1 and uric acid in the detective data (Y) by regression analysis in different clinical stage PR or PF patients using least square convergence [19]. The predicted curve that results in the lowest sum of squares is the best fit. If the fit is robust, then the parameters of the observed curve can be inferred from those of the predicted.
Statistical analysis
For each experiment, the mean and standard error were determined. Statistical differences between groups were assessed by means of Analysis of Variance (ANOVA) from 6 replicate experiments with the post-hoc Dunnett’s test. Statistical significance was considered at P<0.05.
Results
Expression levels of PAI-1 in lung cells
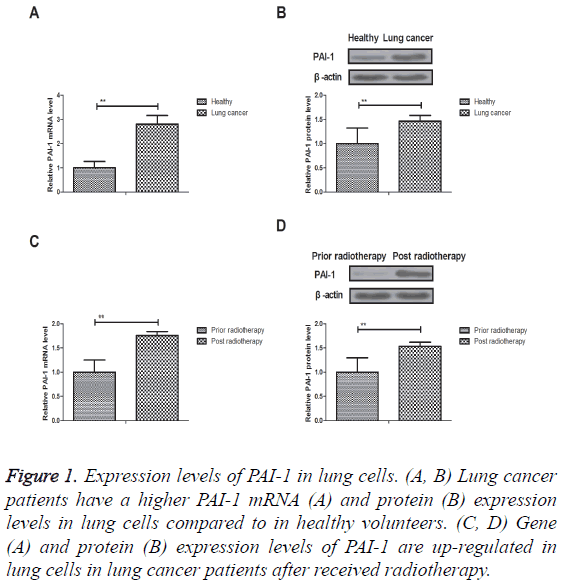
We investigated PAI-1 expression in lung cancer and normal tissues. As shown in Figures 1A and 1B, PAI-1 mRNA and protein expression levels were down-regulated in normal lung cells in healthy volunteers compared to lung cancer patients. We also showed that gene and protein expression levels of PAI-1 were also up-regulated in lung cells in lung cancer patients after received radiotherapy (Figures 1C and 1D). These results suggest that expression levels of PAI-1 were upregulated in lung cells in patients with lung cancer prior and post radiotherapy.
Figure 1: Expression levels of PAI-1 in lung cells. (A, B) Lung cancer patients have a higher PAI-1 mRNA (A) and protein (B) expression levels in lung cells compared to in healthy volunteers. (C, D) Gene (A) and protein (B) expression levels of PAI-1 are up-regulated in lung cells in lung cancer patients after received radiotherapy.
PAI-1 serum levels in patients with lung cancer
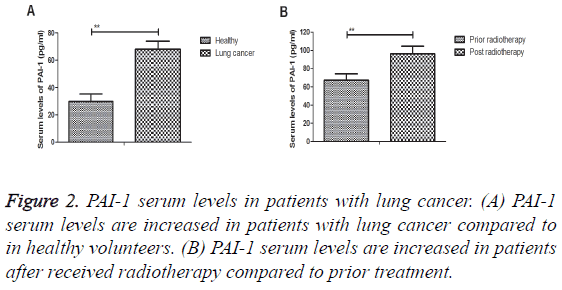
PAI-1 serum levels were analysed in patients with lung cancer compared to healthy volunteers. We observed that PAI-1 serum levels were increased in patients with lung cancer (Figure 2A). We also demonstrated that PAI-1 serum levels were increased in patients after received radiotherapy compared to prior treatment (Figure 2B). These results indicate that PAI-1 serum levels are significantly up-regulated in patients with lung cancer.
Serum levels of PAI-1 in patients with RP
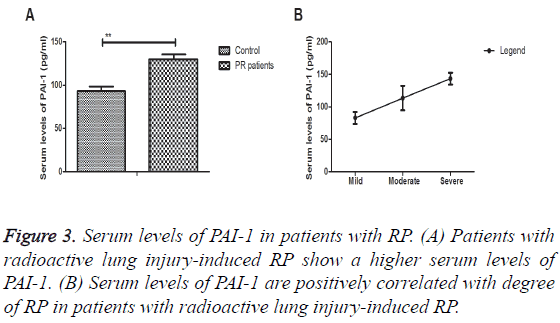
A total of 57 patients with RP were used to analyse serum levels of PAI-1. As shown in Figure 3A, serum levels of PAI-1 were significantly up-regulated in in patients with radioactive lung injury-induced RP. Outcomes demonstrated a positively correlated with degree of RP in patients with radioactive lung injury-induced RP (Figure 3B). These results indicate that serum levels of PAI-1 are associated with radioactive lung injury-induced PR in lung cancer patients.
Serum levels of PAI-1 in patients with RF
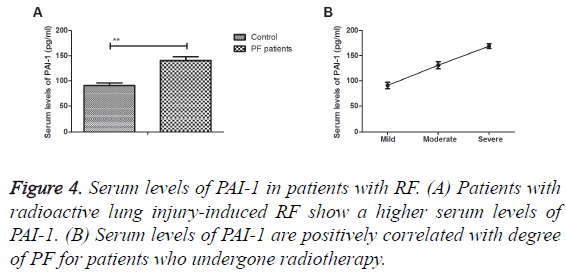
The associations between serum levels of PAI-1 and degree of RF were investigated in lung cancer patients. Results demonstrated that serum levels of PAI-1 were up-regulated in lung injury-induced PF compared to prior treatment (Figure 4A). Serum levels of PAI-1 were positively correlated with degree of PF for patients who undergone radiotherapy (Figure 4B). These results patients with RP presented higher PAI-1 serum levels and positively associated with degree of RP in radioactive lung injury-induced RP.
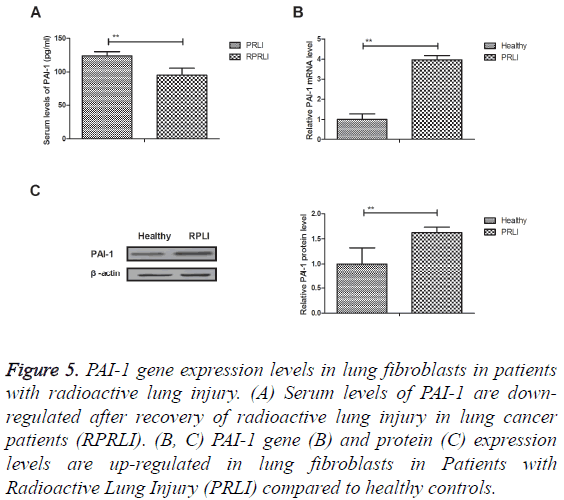
PAI-1 gene expression levels in lung fibroblasts in patients with radioactive lung injury
Results showed that serum levels of PAI-1 were downregulated after recovery of radioactive lung injury in lung cancer patients (RPRLI) (Figure 5A). Outcomes revealed that PAI-1 gene and protein expression levels were up-regulated in lung fibroblasts in Patients with Radioactive Lung Injury (PRLI) compared to healthy controls (Figures 5B and 5C). Collectively, these results suggest that PAI-1 gene and protein expression levels are up-regulated in lung fibroblasts in patients with radioactive lung injury.
Figure 5: PAI-1 gene expression levels in lung fibroblasts in patients with radioactive lung injury. (A) Serum levels of PAI-1 are downregulated after recovery of radioactive lung injury in lung cancer patients (RPRLI). (B, C) PAI-1 gene (B) and protein (C) expression levels are up-regulated in lung fibroblasts in Patients with Radioactive Lung Injury (PRLI) compared to healthy controls.
Discussion
Lung cancer is one of the most common human respiratory tumors that has become a major public health problem and the leading cancer-cause of cancer-related mortality in the world [20]. Evidences have indicated that radiation injury is inevitable after hypofractionated stereotactic radiotherapy for peripheral small lung tumors determined by serial changes on CT [21]. Importantly, elevated PAI-1 serum levels are associated with poor clinical outcomes in pediatric patients with acute lung injury [22]. In this study, we further analysed the changes of PAI-1 expression in lung cells and in serum levels in lung injury patients induced by radiotherapy. We reported the associations between PAI-1 serum levels and degree of RP or RF in lung cancer patients induced by radiotherapy. Outcomes in this study demonstrated that expression levels of PAI-1 were up-regulated in lung cells and serum levels in patients with lung cancer prior and post radiotherapy. Interestingly, findings suggest that PAI-1 serum levels present positively associated with degree of RP and PF in radioactive lung injury-induced RP.
Ochiai et al. have indicated that radiation-induced organizing pneumonia should be considered as one of the late lung injuries after stereotactic body radiotherapy for lung tumors [13]. It is important to be aware the importance of bronchiolitis obliterans organizing pneumonia in the clinical symptoms and radiographic findings after an initiation of steroid therapy [23]. In this study, we observed that lung injuries after radiotherapy significantly affect patients’ living quality. Report also indicated that PAI-1 is an essential component of the pulmonary host response during Pseudomonas aeruginosa pneumonia in mice [16] and PAI-1 gene is associated with multiple organ dysfunction and septic shock in pneumonia induced severe sepsis: prospective, observational, genetic study [24]. We showed that serum levels of PAI-1 are associated with radioactive lung injury-induced PR in lung cancer patients.
Currently, acute exacerbation of subclinical idiopathic pulmonary fibrosis triggered by radiotherapy has been reported in a patient with primary lung cancer and slightly focal honeycombing [25]. Nakajima et al. have suggested radiation can induce pulmonary fibrosis after stereotactic ablative radiotherapy for lung cancer determined by 18F-FDG PET/CT findings [26]. Report also indicated that PAI-1 production is increased in alveolar macrophages in pulmonary fibrosis [27]. We showed that PAI-1 production is up-regulated in patients with RF. Outcomes have demonstrated that patients with RP presented higher PAI-1 serum levels and positively associated with degree of RP in radioactive lung injury-induced RP.
In conclusion, findings in this study have revealed that PAI-1 gene and protein expression levels were up-regulated in lung cancer patients after undergone radioactive lung injury-induced PR or PF. Our outcomes have suggested that PAI-1 serum levels may be regarded as biomarkers to predict radioactive lung injury-induced RP or PF in lung cancer patients. However, further studies are needed to confirm our results in large populations.
References
- Salah S, Tanvetyanon T, Abbasi S. Metastatectomy for extra-cranial extra-adrenal non-small cell lung cancer solitary metastases: systematic review and analysis of reported cases. Lung Cancer 2012; 75: 9-14.
- Corrales-Rodriguez L, Blais N. Lung cancer associated venous thromboembolic disease: a comprehensive review. Lung Cancer 2012; 75: 1-8.
- Futamura Y, Sawa T, Hasegawa T. Review of chemoradiotherapy followed by surgical resection in locally advanced non-small-cell lung cancer. Gan to kagaku ryoho. Cancer Chemother 2012; 39: 2390-2392.
- Gamble JF, Nicolich MJ, Boffetta P. Lung cancer and diesel exhaust: an updated critical review of the occupational epidemiology literature. Crit Rev Toxicol 2012; 42: 549-598.
- Singh N, Jindal A, Behera D. Erlotinib usage after prior treatment with gefitinib in advanced non-small cell lung cancer: A clinical perspective and review of published literature. World J Clin Oncol 2014; 5: 858-864.
- Sekiguchi Y, Shimada A, Imai H. Patient with refractory multiple myeloma developing eosinophilia after lenalidomide treatment and lung cancer 9 months later: case report and review of the literature. Ind J Hematol Blood Transfusion 2014; 30: 264-270.
- Toy E, Macbeth F, Coles B, Melville A, Eastwood A. Palliative thoracic radiotherapy for non-small-cell lung cancer: a systematic review. Am J Clin Oncol 2003; 26: 112-120.
- Taremi M, Hope A, Lindsay P, Dahele M, Fung S. Predictors of radiotherapy induced bone injury (RIBI) after stereotactic lung radiotherapy. Radiat Oncol 2012; 7: 159.
- Xing J, Li JB, Yu JM. Relationship of dose-volume histogram parameters and computed tomography grading of radiation-induced lung injury in patients with non-small cell lung cancer treated by three-dimensional conformal radiotherapy. Zhonghua Zhong Liu Za Zhi Chinese J Oncol 2008; 30: 676-681.
- Cella L, Liuzzi R, Davino V. Pulmonary damage in Hodgkins lymphoma patients treated with sequential chemo-radiotherapy: Predictors of radiation-induced lung injury. Acta Oncol 2014; 53: 613-619.
- Hong ZY, Lee HJ, Choi WH, Lee YJ, Eun SH. A preclinical rodent model of acute radiation-induced lung injury after ablative focal irradiation reflecting clinical stereotactic body radiotherapy. Radiat Res 2014; 182: 83-91.
- Kim H, Bae H, Lee MY. Analysis of predictive factors for lung injury after forward-planned intensity-modulated radiotherapy in whole breast irradiation. J Breast Cancer 2014; 17: 69-75.
- Ochiai S, Nomoto Y, Yamashita Y. Radiation-induced organizing pneumonia after stereotactic body radiotherapy for lung tumor. J Rad Res 2015; 56: 904-911.
- He J, Deng L, Na F, Xue J, Gao H, Lu Y. The association between TGF-beta1 polymorphisms and radiation pneumonia in lung cancer patients treated with definitive radiotherapy: a meta-analysis. PloS One 2014; 9: 91100.
- Bhattacharyya T, Bhattacharjee A. Competing risk: An illustration with aspiration pneumonia in head and neck cancer patients undergoing radical radiotherapy: A biostatisticians perspective. Ind J Cancer 2014; 51: 406-409.
- Goolaerts A, Lafargue M, Song Y. PAI-1 is an essential component of the pulmonary host response during Pseudomonas aeruginosa pneumonia in mice. Thorax 2011; 66: 788-796.
- Osterholzer JJ, Christensen PJ, Lama V. PAI-1 promotes the accumulation of exudate macrophages and worsens pulmonary fibrosis following type II alveolar epithelial cell injury. J Pathol 2012; 228: 170-180.
- Perez-Callejo D, Romero A, Provencio M, Torrente M. Liquid biopsy based biomarkers in non-small cell lung cancer for diagnosis and treatment monitoring. Transl Lung Cancer Res 2016; 5: 455-465.
- Hayes AF, Rockwood NJ. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav Res Ther 2016.
- Magnuson WJ, Yeung JT, Guillod PD, Gettinger SN, Yu JB, Chiang VL. Impact of deferring radiation therapy in patients with epidermal growth factor receptor-mutant non-small cell lung cancer who develop brain metastases. Int J Rad Oncol Biol Phys 2016.
- Takeda T, Takeda A, Kunieda E. Radiation injury after hypofractionated stereotactic radiotherapy for peripheral small lung tumors: serial changes on CT. AJR. Am J Roentgenol 2004; 182: 1123-1128.
- Sapru A, Curley MA, Brady S, Matthay MA, Flori H. Elevated PAI-1 is associated with poor clinical outcomes in pediatric patients with acute lung injury. Intensive Care Med 2010; 36: 157-163.
- Hojo S, Yoshioka S, Toyoda Y. Two cases of bronchiolitis obliterans organizing pneumonia (BOOP) induced radiotherapy after surgery of breast cancer. Gan to kagaku ryoho. Cancer Chemother 2010; 37: 2781-2783.
- Madach K, Aladzsity I, Szilagyi A. 4G/5G polymorphism of PAI-1 gene is associated with multiple organ dysfunction and septic shock in pneumonia induced severe sepsis: prospective, observational, genetic study. Crit Care 2010; 14: 79.
- Takeda A, Enomoto T, Sanuki N. Acute exacerbation of subclinical idiopathic pulmonary fibrosis triggered by hypofractionated stereotactic body radiotherapy in a patient with primary lung cancer and slightly focal honeycombing. Rad Med 2008; 26: 504-507.
- Nakajima N, Sugawara Y, Kataoka M. Differentiation of tumor recurrence from radiation-induced pulmonary fibrosis after stereotactic ablative radiotherapy for lung cancer: characterization of 18F-FDG PET/CT findings. Ann Nucl Med 2013; 27: 261-270.
- Ueno M, Maeno T, Nomura M. Hypoxia-inducible factor-1alpha mediates TGF-beta-induced PAI-1 production in alveolar macrophages in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2011; 300: 740-752.