ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Case Report - Biomedical Research (2017) Volume 28, Issue 22
Post-traumatic necrotizing fasciitis and sepsis shock caused by Aeromonas hydrophila: A case report and summary of related reports
Jian Sun, Xin Tian*, Weijun Fang, Jinwei Huang, Tao Zhang and Jiasheng Ding
Department of Critical Care Medicine, Lishui Center Hospital, Lishui, Zhejiang, China
Accepted date: October 16, 2017
Necrotizing Fasciitis (NF) and sepsis shock is a rare disease in human caused by bacteria infection which is detrimental to patients. We report the diagnosis, progress, treatment and prognosis of patients with NF and sepsis shock caused by Aeromonas hydrophila (A. hydrophila). A 64 y old man was admitted for Gustilo type-iii B open fracture of the left tibia and fibula. Debridement and exopexy were carried out immediately after which he received liquid infusion and empirical antibiotic therapy in ICU. However, the condition became worse 24 h later and large extensive necrosis of the fascia and surrounding tissue was observed and the patients was in hypodynamic septic shock. Therefore, the infected limb was amputated and in the meantime, culture test for the necrotic tissue revealed presence of A. hydrophila and Klebsiella. Piperacillin and Tazobactam were applied to the patient for eliminating klebsiella and A. hydrophila respectively. Eleven weeks after admission, all blood biochemical parameter recover to normal and the patient was discharged. We present a rare case of A. hydrophila NF and sepsis shock in an elderly patient and suggest that resecting debridement of necrotic fascia and even limb-amputation is required for successful treatment.
Keywords
Necrotizing fasciitis, Sepsis shock, Aeromonas hydrophila, Excisional debridement, Amputation
Introduction
Aeromonas hydrophila is gram-negative bacteria and is member of the family Vibrionaceae. It is mainly found in sewage, fresh or brackish water, soil, tap water, and nonfecal organic materials [1]. They can cause variety of infections including gastrointestinal bleeding, skin and soft tissue infections, sepsis and other syndromes [2]. Thus, the understanding of the feature and treatments will be beneficial for treating them in clinic application. Necrotizing Fasciitis (NF) is a rapidly progressing fatal lesion in the skin and muscle tissue for mixed infection [3]. There are sporadic reports of cases of sepsis or necrotizing fasciitis caused by A. hydrophila but no case of severe infection secondary to trauma. Here we present the clinical course of a patient suffering necrotizing fasciitis and sepsis caused by A. hydrophila after traumatic injury. Furthermore, we used the PubMed database to search for recent case reports of infections caused by A. hydrophila.
Case Report
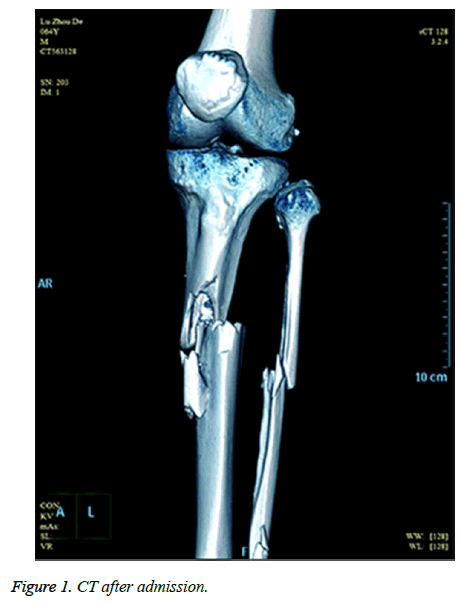
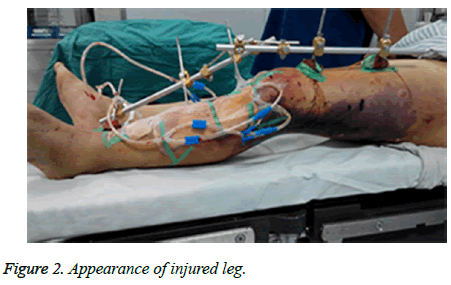
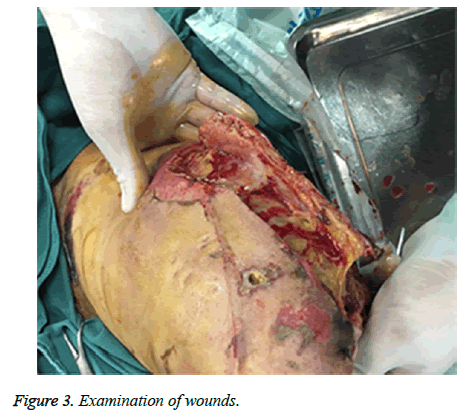
A sixty-four-year-old man with no relevant past medical history and no previous exposure contact with aquatic animals dropped from a motorcycle in a ditch filled with stagnant water. On arriving at hospital, he was hemodynamically stable with Gustilo type-iii B open fracture of the left tibia and fibula without neurovascular impairment (Figure 1). The left leg with traumatic injury accepted debridement and exopexy immediately (Figure 2). After operation, patient was admitted to Intensive Care Unit (ICU) and received normal saline and empirical antibiotics (amoxicillin/clavulanic acid 4 g × 4 and clindamycin 600 mg × 3) on the first day in ICU. However, 24 h later, the lesions became worse as foul smell and signs of necrotic-haemorrhagic bullae like disseminated soft-tissue infection were evident (Figure 3). Surgical exploration revealed extensive necrosis of the fascia and surrounding tissues in the wounds. Furthermore, tissue samples were dissected and wound cultures were prepared for bacteriological examination. The patient was febrile (temperature of 38.3°C) with heart pulse 128 beats/min, respiratory rate 30/min and blood pressure 83/56 mmHg. He was in hypodynamic septic shock and remained hypotensive despite administration of norepinephrine, milrinone, severe respiratory failure, signified by a PaO2:FiO2 ratio of 88. Laboratory results showed the following: white blood cell count 18000 cells/mm3 (normal range: 5,000-10,000 cells/mm3, 97.7% neutrophils, 2.3% lymphocytes), hemoglobin 7.6 g/dL, hematocrit 26%, platelet count 30,000/mm3, C-reactive protein above 200 mg/L, procalcitonin 10.96 ng/ml, serum creatinine kinase 807 U/L, serum myohemoglobin concentration 6577.0 ng/ml, urea nitrogen 16.9 mmol/L, serum creatinine 177 μmol/L, BNP 2882.3 pg/ml, Glu 5.7 mmol/L, total bilirubin 197.4 μmol/L, direct bilirubin 146.2 μmol/L, indirect bilirubin 51.2 μmol/L and PT/APTT/INR: 17.0/41.2/2.16.
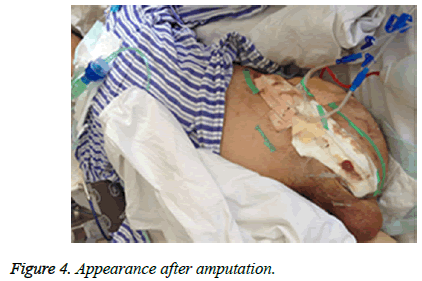
Patients’ family initially refused amputation at higher level and the patient was brought to the operating room for revisiting the left leg, including debridement, and Vacuum Sealing Drainage (VSD). Imipenem 1.0 g × 4 and tigecycline 50 mg × 2 were administrated instead for antibiotic therapy after sepsis shock occurrence. After surgery, the patient remained febrile, hypotensive and oliguric renal failure with a greater demand for vasoactive medications and rising lactate concentration (from 2 mmol/l to 5 mmol/l). On the fact of obvious extremis, the family agreed and the patient received middle of left leg amputation below hip and VSD irrigation. During the surgery the patient received multiple units of packed red blood cells and fresh frozen plasma. The patient had progressive multiorgan failure and deteriorating renal function requiring hemodialysis. After 72 h, Culture test confirmed the presence of A. hydrophila and Klebsiella in wound tissues but not in blood and sputum samples. A. hydrophila is sensitive to cefuroxime, cefotaxime, ciprofloxacin, gentamicin and trimethoprim but resistant to ampicillin, while Klebsiella is sensitive to ciprofloxacin, imipenem, amikacin, piperacillin and tazobactam but resistant to cefotaxime, ceftriaxone and ampicillin. Antimicrobial susceptibility was tested in laboratory. piperacillin and tazobactam 4.5 g × 6 were administrated instead for antibiotic therapy based on culture test and antimicrobial susceptibility tests. Two weeks after, Klebsiella and A. hydrophila were eliminated two and three weeks, respectively later. the patient received 7 times VSD irrigation and debridement to the amputation stump until all necrotic tissue was debrided (Figure 4). In total, the patient was admitted for eleven weeks (including nine weeks in ICU) and white cell count, CRP and Procalcitonin turned normal. He can walk with crutches and move with wheelchair for short distance. The patient was discharged and followed-up at regular intervals.
Based on available literatures from PubMed Database, clinical parameters of 10 relevant cases were summarized in Table 1. There were 8 males and 2 females, with a mean age of 59 y old (30-72). Among them, eight cases suffered systemic infection with predisposing factors such as hemodialysis, immunosuppression, diabetes and chronic liver disease. Five cases were without contact history with any aquatic animal, so the infection source could be stagnant water with microorganism and the infection could occur via traumatic penetration through tiny breaks in skin. All cases had been admitted in ICU, with mean duration of 30 d (3-63). Besides A. hydrophila, other pathogens were found in 4 cases. Adequate antibiotics were initially administered for empiric therapy and excisional debridement of the necrotic fascia or immediate limb amputation was performed early to all patients. However, 8 patients recovered while 2 patients died finally.
| Patient no. | Patient 1 [13] | Patient 2 [14] | Patient 3 [15] | Patient 4 [16] | Patient 5 [17] |
|---|---|---|---|---|---|
| Sex, age | M, 55 | M, 58 | M, 60 | M, 63 | M, 61 |
| Infection site | Blood | Blood | Wound | The lower left leg | Epiglottitis |
| Dialysis catheter | Wound | Neck soft tissue | |||
| Rectum | |||||
| Type of infection | Sepsis | Necrotizing, fasciitis | Necrotizing, fasciitis | Ecthyma gangrenosum | Necrotizing fasciitis |
| Soft tissue infections | |||||
| Disease/risk factors | Kidney-failure, hemodialysis, immunosuppressive agents |
None | Cirrhosis | Diabetes, obese | Cirrhosis |
| Type-two diabetes | Hypertensive cardiopathy | ||||
| Contacting, history/rural setting | None | Stagnant water | None | Stagnant water in well | None |
| ICU-days | 14 | 49 | 3 | 60 | 21 |
| Other pathogens | Pseudomonas aeruginosa | None | None | None | |
| Initial drug (antibiotics) | Piperacillin | Clindamycin | Piperacillin | Meropenem | Ceftriaxone, |
| Tazobactam | Cefuroxime | Tazobactam | Tigecycline | Clindamycin, | |
| Ciprofloxacin | |||||
| Operation/treatment | Dialysis catheter removal | Amputation followed by right hip | Fasciectomies | Hyperbaric oxygen chamber therapy | Tracheostomy, |
| Debridements | Skin grafting | Fasciotomy, | |||
| Surgery | Debridement | ||||
| Outcome | Recovered | Died | Died | Recovered | Recovered |
| Patient no. | Patient 6 [18] | Patient 7 [19] | Patient 8 [20] | Patient 9 [21] | This report |
| Sex, age | F, 72 | M, 66 | M, 30 | F, 60 | M, 64 |
| Infection site | Blood, bile | The right foot | Lung | Breast | Left leg |
| Type of infection | Cholecystitis; blood stream infection | Necrotizing, fasciitis | Fulminant pneumonia | Cellulitis, abscess | Necrotizing, fasciitis |
| Disease/risk factors | Rheumatoid arthritis, acute suppurative cholangitis | Diabetic gangrene, renal failure, haemodialysis, liver cirrhosis, hepatocellular carcinoma | Cardiac arrest, aspiration pneumonia | Breast Reconstruction, leech therapy | None |
| Contacting, history/rural setting | Endoscopic retrograde cholangiopancreatography | None | Stagnant water | None | Stagnant water |
| ICU-days | 18 | 49 | 27 | 3 | 63 |
| Other pathogens | Klebsiella pneumoniae | None | Streptococcus | None | Klebsiella pnuemoniae, Enterococcus faecalis |
| Initial drug (antibiotics) | Piperacillin, tazobactam | Clindamycin, cefuroxime, minocycline | Piperacillin, tazobactam, levofloxacin | Ciprofloxacin, vancomycin, aztreonam | Tienam, tigecycline |
| Operation/treatment | Laparoscopic cholecystectomy | Right, leg amputation | ECMO | Implant removed, debridement | Amputation followed by left hip, VSD debridement |
| Outcome | Recovered | Recovered | Recovered | Recovered | Recovered |
Table 1. Clinical parameters of patients with A. hydrophila infection.
Discussion
Aeromonas hydrophila are gram-negative bacilli commonly found in aquatic environments [4], such as stagnating, streaming surface water, wells and even swimming pool [5]. They can also cause diseases in humans including gastroenteritis, water-associated traumatic wound infections including necrotizing fasciitis, meningitis, peritonitis, and bacteremia [6]. Necrotizing Fasciitis (NF) is a deadly necrotic inflammation of skin, soft-tissues, and muscle bundles [7]. The mechanism has been found to be accounted for A. hydrophila infection, which is injury of soft skin tissue, and leading to sepsis and secondary necrotizing fasciitis [8].
We analysed A. hydrophila infection in soft skin tissue in the present case and summarized that in previous reported cases. Most of these patients can be considered immunosuppressed to some degree because of certain medical conditions, such as hemodialysis, immunosuppression, diabetes and chronic liver disease. The mechanism responsible for the severe course of A. hydrophila infection involves production of highly toxic exotoxins, such ascytolysin, hemolysin, cytotoxic enterotoxin, and cholera toxin-like factor [9]. In the 10 cases summarized, only 5 cases (half of ten) were infected through wound exposure and necrotizing fasciitis attributable to A. hydrophila were believed to develop only in an immunocompromised host. And in this sense, sepsis shock, a most invasive form of infection, may be also as a result of immunocompromising in the presence of infection.
Necrotizing fasciitis and sepsis caused by A. hydrophila were associated with high mortality, thus early surgical intervention was essential [10]. Some investigators have reported that A. hydrophila infection can cause severe necrotizing fasciitis and myonecrosis. Early and aggressive surgical intervention must be applied immediately after necrotizing fasciitis is diagnosed as patients could die due to delayed surgical removal of necrotic lesions (including amputation) [11,12]. For our patient, broad-spectrum antibiotics were administered within one hour after sepsis was identified and aggressive multimodality treatments together contributed to patient's prognosis. Including our patients, among the ten cases summarized patients were benefit to receiving excisional debridement of the necrotic fascia or early limb amputation.
Conclusions
In conclusion, together with previous study, a general treatment against A. hydrophila infection can be arrived. And the empiric Antibiotics which should be used started in cases of suspected necrotizing fascitis before treatment. Firstly, rapidly progressing of soft-tissue infection could indicate A. hydrophila infection. Secondly, aggressive antibiotic drugs were essential to administered for empiric therapy. Thirdly, early diagnosis of all forms of A. hydrophila infection is of significance, especially in the case of necrotizing fasciitis and sepsis. Fourthly, amputation, debridement and VSD irrigation in combination with appropriate antibiotic treatment should be applied for good prognosis.
References
- Tsai Y, Shen S, Yang T, Chen P, Huang K, Lee MS. Monomicrobial necrotizing fasciitis caused by Aeromonas hydrophila and Klebsiella pneumoniae. Med Princip Pract 2015; 24: 416-423.
- Yumoto T, Ichiba S, Umei N, Morisada S, Tsukahara K, Sato K, Ugawa T, Ujike Y. Septic shock due to Aeromonas hydrophila bacteremia in a patient with alcoholic liver cirrhosis: a case report. J Med Case Rep 2014; 8: 402.
- Ponnusamy D, Kozlova EV, Sha J, Erova TE, Azar SR, Fitts EC, Kirtley ML, Tiner BL, Andersson JA, Grim CJ, Isom RP, Hasan NA, Colwell RR, Chopra AK. Cross-talk among flesh-eating Aeromonas hydrophila strains in mixed infection leading to necrotizing fasciitis. Proc Nat Acad Sci 2016; 113: 722-727.
- Igbinosa IH, Igumbor EU, Aghdasi F, Tom M, Okoh AI. Emerging Aeromonas species infections and their significance in public health. Sci World J 2012; 2012: 1009-1014.
- Julia MM, Vicente VA, Gene GA, Gonzalez-Ensenat MA. Aeromonas hydrophila folliculitis associated with an inflatable swimming pool: mimicking Pseudomonas aeruginosa infection. Pediatr Dermatol 2009; 26: 601-603.
- Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev 2010; 23: 35-73.
- Sever R, Lee GA, Steinberg E, Soffer D. Trauma with a touch of fresh water: necrotizing fasciitis caused by Aeromonas hydrophilia after a motorcycle accident. Am Surg 2013; 79: 326-328.
- Corredoira JM, Ariza J, Pallares R, Carratala J, Viladrich PF, Rufí G, Verdaguer R, Gudiol F. Gram-negative bacillary cellulitis in patients with hepatic cirrhosis. Eur J Clin Microbiol Infect Dis 1994; 13: 19-24.
- Grim CJ, Kozlova EV, Ponnusamy D, Fitts EC, Sha J, Kirtley ML, van Lier CJ, Tiner BL, Erova TE, Joseph SJ, Read TD, Shak JR, Joseph SW, Singletary E, Felland T, Baze WB, Horneman AJ, Chopra AK. Functional genomic characterization of virulence factors from necrotizing fasciitis-causing strains of Aeromonas hydrophila. Appl Env Microbiol 2014; 80: 4162-4183.
- Monaghan SF, Anjaria D, Mohr A, Livingston DH. Necrotizing fasciitis and sepsis caused by Aeromonas hydrophila after crush injury of the lower extremity. Surg Infect (Larchmt) 2008; 9: 459-467.
- Baruah FK, Ahmed NH, Grover RK. Surgical site infection caused by Aeromonas hydrophila in a patient with underlying malignancy. J Clin Diagn Res 2015; 9: 1-2.
- Markov G, Kirov G, Lyutskanov V, Kondarev M. Necrotizing fasciitis and myonecrosis due to Aeromonas hydrophila. Wounds 2007; 19: 223-226.
- Khalil MAM, Rehman A, Kashif WU, Rangasami M, Tan J. A rare case of Aeromonas hydrophila catheter related sepsis in a patient with chronic kidney disease receiving steroids and dialysis: a case report and review of Aeromonas infections in chronic kidney disease patients. Case Rep Nephrol 2013; 2013: 1-5.
- Borger van der Burg BL, Bronkhorst MW, Pahlplatz PV. Aeromonas hydrophila necrotizing fasciitis. A case report. J Bone Joint Surg Am 2006; 88: 1357-1360.
- Liao KC, Yen PT, Liu C. Necrotizing fasciitis caused by inconspicuous infection of Aeromonas hydrophila in an immunocompromised host. J Surg Case Rep 2010; 2010: 2.
- Avolio M, La Spisa C, Moscariello F, De Rosa R, Camporese A. Aeromonas hydrophila ecthyma gangrenosum without bacteraemia in a diabetic man: the first case report in Italy. Infez Med 2009; 17: 184-187.
- Minnaganti VR, Patel PJ, Iancu D, Schoch PE, Cunha BA. Necrotizing fasciitis caused by Aeromonas hydrophila. Heart Lung 2000; 29: 306-308.
- Okumura K, Shoji F, Yoshida M, Mizuta A, Makino I, Higashi H. Severe sepsis caused by Aeromonas hydrophila in a patient using tocilizumab: a case report. J Med Case Rep 2011; 5: 499.
- Furusu A, Yoshizuka N, Abe K, Sasaki O, Miyazaki K, Miyazaki M, Hirakata Y, Ozono Y, Harada T, Kohno S. Aeromonas hydrophila necrotizing fasciitis and gas gangrene in a diabetic patient on haemodialysis. Nephrol Dial Transplant 1997; 12: 1730-1734.
- Issa N, Napolitano LM. Aeromonas pneumonia in a trauma patient requiring extracorporeal membrane oxygenation for severe acute respiratory distress syndrome: case report and literature review. Surg Infect 2011; 12: 241-245.
- Patel KM, Svestka M, Sinkin J, Ruff P. Ciprofloxacin-resistant Aeromonas hydrophila infection following leech therapy: A case report and review of the literature. J Plast Reconstr Aesthet Surg 2013; 66: 20-22.