ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 9
Preparation of oligopeptides from corn gluten meal by two enzymes at one step using response surface methodology and investigation of their antifatigue activities
1Institute of Agro-food Science and Technology, Shandong Academy of Agricultural Science, Jinan, China
2College of Biological Science and Technology, University of Jinan, Jinan, China
#These authors contributed equally to this work
- *Corresponding Author:
- Naxin Sun
College of Biological Science and Technology
University of Jinan, China
Accepted date: January 31, 2017
The corn gluten meal, byproduct through the corn starch industry, was hydrolyzed with the combination of alkaline protease and papain to produce oligopeptides with high Fisher-values. Response surface methodology was applied to optimize the hydrolysis conditions, including reaction temperature, pH, enzyme to substrate ratio, and percentage papain added. The highest degree of hydrolysis (29.51%) was obtained at a reaction temperature of 55?C, a reaction pH at 11.10, an enzyme to substrate ratio of 0.85, and percentage of papain added at 20%, which was in agreement with the predicted value (30.84%) estimated by response surface methodology within a 95% confidence interval. In addition, the final product was the mixture of oligopeptides with relatively high content of branched-chain amino acids and in vivo the effect on physical fatigue was investigated by using a loaded swimming test, along with the determination of blood lactic acid, blood urea nitrogen, liver glycogen and muscle glycogen contents. The data showed that the oligopeptides could extend exhaustive swimming times of mice, as well as increase liver and muscle glycogen contents and decrease blood lactic acid contents. These results support that the oligopeptides, prepared from corn proteins using the combination of two proteases, have an anti-physical fatigue effect.
Keywords
Corn gluten meal, Alkaline protease, Papain, Response surface methodology, Branched-chain amino acid, Anti-fatigue.
Introduction
Physical fatigue can be described as an inability to translate the motor drive into an expected force due to intense muscle activity [1]. It is a complex physiological and biochemical process, which acts as a protective mechanism for the human body in response to life threatening over-exhaustion. However, at present fatigue has been becoming a common social problem among people who live under enormous stressful life and has brought about much negative impact on physical function [2-4]. Accordingly, the rising incidence of disease related to physical fatigue continues to challenge scientists in addressing various agents, including food source and non-food source, towards the development of anti-fatigue product [5-8].
Corn Gluten Meal (CGM), a byproduct of the corn starch industry, contains 60% protein and thus is a very economic protein source. However, because of its special taste, low water solubility and shortage of some essential amino acids, CGM is used mainly for animal feed [9,10]. In spite of this, based on its biochemical structure, low price and high abundance, trials for improving the bioavailability of CGM have been conducted in order to expand its applications. Among them, the most widely studied is the preparation of functional oligopeptides or free amino acids through enzymatic hydrolysis. The hydrolysates prepared from CGM have exhibited various profitable effects, such as radical scavenging activity and lipid peroxidation inhibitory effects [9,11-13], anti-fibrotic activity [14], and protective effects on alcohol-induced liver damage with chronic alcohol consumption [15]. As it has been known that there is a close relationship between the structures or characteristic amino acid compositions of peptides and their biological functionalities. Some special amino acids, such as Branched-Chain Amino Acids (BCAAs), have been getting special attention owing to their possible role in energy metabolism and the longevity of species ranging from unicellular organisms to mammals [16]. Sami demonstrated that CGM has a relatively higher level of BCAAs and a lower level of Aromatic Amino Acids (AAAs) than wheat gluten meal [17], thus the oligopeptides with high Fischer index (a molar ratio of BCAA and AAA) might be expected to be obtained from the hydrolysates of CGM. Furthermore, there is limited information on the anti-fatigue activity of the oligopeptides with high Fisher index prepared from the corn protein.
Alkaline proteinase, papain, and neutral proteinase are the commonly applied proteases to prepare the low weight peptides from CGM, and stepwise hydrolysis by two enzymes is often necessary to obtain the hydrolysates with a high degree of protein hydrolysis [18]. The fractional hydrolysis with two enzymes has the advantage of providing the optimum enzymatic condition by controlling enzymatic hydrolysis condition suitable for each enzyme, respectively. However, the relevant steps are relatively more complicated and would spend longer time compared with one step enzymatic hydrolysis containing two enzymes. Hence, the present work investigated the feasibility and the optimal conditions of applying the compounding enzymes containing alkaline protease and papain to hydrolyse the CGM for the preparation of Oligopeptides (CPs) with high Fisher-values, and in vivo anti-fatigue activity of the CPs was also examined through the loaded swimming exercise of mice.
Materials and Methods
CGM and enzymes
The corn proteins were obtained from corn gluten meal provider LuZhou Co. (Shandong, China). The crude protein content was 80.15%, as determined by the Kjeldahl method.
Alkaline protease (EC 3.4.21.14) and papain (EC 3.4.22.2) used for the hydrolysis of CGM were provided by Sigma Int.
Preparation of hydrolysis and degree of hydrolysis
All hydrolysis reactions were performed in 250 ml Erlenmeyer flasks, containing 3 g of CGM samples with Glycine-NaOH buffer (50 ml). Reactions were carried out in triplicate in a thermostatically controlled water bath, a pH electrode, and a mixer shaft for addition of alkali. Enzymes were added and temperature was controlled during the hydrolysis reaction and pH was monitored by the pH stat method using automatic Mettler DL 25 titration unit [19]. Hydrolysis was continued with alkaline protease or/and papain for 120 min, after which the reaction was stopped by heating to 90°C for 5 min. Samples were cooled and then centrifuged at 16000 g for 10 min at 4°C. The supernatants were collected and used for further absorption experiments.
The Degree of Hydrolysis (DH) is defined as the ratio (expressed as (%)) between the number of hydrolyzed peptide bonds over the total number of bonds available for hydrolysis which was measured by the pH-stat method. DH was calculated from the following Equation 1.
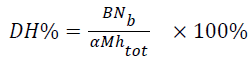 →(1)
→(1)
B-consumption of NaOH for hydrolysis (ml); Nb-the concentration of NaOH; α-average degree of dissociation of the α-NH groups (CGM is 1.01); M-total mass of protein (g) in the reaction mixture; htot-total number of peptide bonds in the test protein substrate (CGM is 9.2 mol/g).
Experimental design
To optimize hydrolysis conditions, the DH was considered as the response variable and four reaction parameters were optimized using Response Surface Methodology (RSM). The One-Factor-At-a-Time method (OFAT) experiments were conducted first to choose the most critical factors and their reasonable ranges (fixed level of four factors were E/S ratio=0.6, time at 2 h, pH=10 and temperature=50˚C). The range and center point values of the four independent variables presented in Table 1 were based on the results of preliminary experiments. The Reaction temperature (X1), pH (X2), Enzyme/ substrate ratio (w/w) (X3) and the percentage (mass) of papain added (X4) were chosen for independent variables, and the results of the effect of each variable on the response were presented in Table 2. The RSM was comprised of 29 treatments including 24 factorial points, eight axial points (α=1.41) and five replicates at the center points. Experimental runs were randomized to minimize the effects of unexpected variability in the observed responses. DH is used as the response for the combination of the independent variables as shown in Table 2. The effect of each independent variable was eventually examined on the surface of this response. The model proposed for the response is presented in Equation 2.
| Variable | Code | Code level | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| Temperature(˚C) | X1 | 50 | 55 | 60 |
| pH | X2 | 10.5 | 11.0 | 11.5 |
| E/S | X3 | 0.6 | 0.8 | 1.0 |
| Percentage papain added(%) | X4 | 15 | 25 | 35 |
Table 1: Factors and levels in the RSM experimental design.
| No. | Coded levels of variable | DH (%) | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Y | |
| 1 | 0 | 1 | -1 | 0 | 27.44 |
| 2 | 1 | 0 | -1 | 0 | 28.52 |
| 3 | 0 | -1 | 0 | 1 | 28.34 |
| 4 | 1 | 1 | 0 | 0 | 27.47 |
| 5 | -1 | 0 | 0 | -1 | 27.65 |
| 6 | 0 | 0 | 0 | 0 | 32.34 |
| 7 | 0 | 0 | 0 | 0 | 31.44 |
| 8 | -1 | 1 | 0 | 0 | 29.26 |
| 9 | -1 | 0 | 1 | 0 | 26.23 |
| 10 | 0 | 0 | 0 | 0 | 31.42 |
| 11 | 0 | 0 | 0 | 0 | 29.26 |
| 12 | 0 | -1 | 0 | -1 | 28.7 |
| 13 | -1 | -1 | 0 | 0 | 26.39 |
| 14 | 0 | 0 | 1 | -1 | 26.1 |
| 15 | 1 | 0 | 1 | 0 | 26.9 |
| 16 | 0 | 1 | 0 | 1 | 27.98 |
| 17 | 0 | 0 | -1 | -1 | 27.44 |
| 18 | 1 | 0 | 0 | 1 | 29.26 |
| 19 | 1 | -1 | 0 | 0 | 28.34 |
| 20 | 0 | 0 | -1 | -1 | 30.13 |
| 21 | 0 | 0 | 0 | 0 | 29.26 |
| 22 | -1 | 0 | 0 | 1 | 28.19 |
| 23 | 0 | -1 | -1 | 0 | 27.98 |
| 24 | -1 | 0 | -1 | 0 | 26.9 |
| 25 | 0 | 1 | 1 | 0 | 29.06 |
| 26 | 0 | 0 | 1 | 1 | 28.79 |
| 27 | 1 | 0 | 0 | -1 | 30.13 |
| 28 | 0 | -1 | 1 | 0 | 29.33 |
| 29 | 0 | 1 | 0 | -1 | 29.42 |
Table 2: Experimental design in RSM studies by using four independent variables showing observed DH.
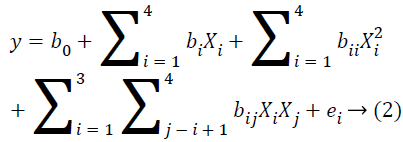
Where y is the response variable or the degree of hydrolysis; b0 is constant; bi represents the regression coefficient for linear effect; bii represents the quadratic coefficient; bij is the interaction coefficient and ei is the random error [20].
Preparation of corn oligopeptides (CPs) with high Fischer ratio
Corn gluten meal was crushed and sieved using an 80 mesh sieve. The permeate was collected and dispersed in the corresponding buffer to obtain a 60 g/L protein slurry, then the mixture was hydrolyzed under the specified conditions for 2 h under continuous stirring. At the end of the hydrolysis period the mixture was heated to 90˚C for 10 min to inactivate the protease. After the hydrolysate cooled down to the room temperature, 1 M hydrochloric acid was used to adjust the pH to 2.0. Then, 60 mesh activated charcoals were added at the amount of 12% (W/V) at room temperature for 3 h under continuous stirring, for it has a higher absorption capacity to the AAA than to the BCAA (data not shown) (Yantai, China). After centrifuged at 1000 Xg for 10 min and concentrated by rotary evaporation, the solution was then fractionated using ultrafiltration with Molecular Weight Cut-Off (MWCO) membranes of 3 kDa (Pall, America). The permeate was obtained and desalinated by adding ion exchange resin at the amount of 10% (W/V) for 2 h under continuous mixing, then the solution was lyophilized and stored at -20˚C for the following research.
Treatment of animals
Kunming mice (8 weeks old, males, SPF, 18-22 g body weight) were purchased from the Shandong Laboratory Animal Center (Jinan, China), and housed in stainless steel wire-bottomed cages with free access to water and feed at 22 ± 2˚C with a 12 h/12 h light/dark. All animals were cared humanely, and the studies reported here have been carried out in accordance with the principles for the Humane Treatment of Animals set by the Association of Laboratory Animal Sciences at College of Biological Science and Technology, Jinan University.
Animals grouping and treatment
40 male mice were randomly divided into 4 groups with 10 in each group. All were administered orally and daily for 30 days. Group Control received isotonic saline solution as control; Group Low, Med and High obtained 0.1, 0.3 and 0.5 mg/kg body weight of CPs solutions, respectively. The mice were made to swim for 15 min three times a week to accustom them to swimming. The exhaustive swimming exercise was conducted after 1 h of the last gavage on day 30. The mice were submitted to a forced swimming test to determine the effect of CPs on physical fatigue.
Forced swimming test
Physical fatigue was induced by forcing animals to swim until exhaustion. The mice were placed in a tank with 30 cm deep water in it. Water temperature was maintained at 25 ± 1˚C. The mice were loaded with a steel washer weighing approximately 5% of their body weight attached to the tails. The mice swam until exhaustion, which is defined as the moment when the mouse was not able to maintain its nose out of water for more than 10 s [7], and the swimming time was recorded.
Biochemical parameters assay
After the forced swimming test, the mice were allowed to rest for 1 h. They were then taken out and the blood was collected through eyeballs to prepare the serum for use, while the liver and gastrocnemius muscle were collected to be made into 10% homogenates with normal saline at 4°C as soon as possible. The Blood Lactic Acid (BLA), Blood Urea Nitrogen (BUN), and liver glycogen and muscle glycogen were examined according to the kit instructions (Jiancheng Bio., Nanjing, China).
Statistical analysis
The data are expressed as means ± S.D. Statistical comparisons were compared by one-way Analysis of Variance (ANOVA). The results were considered statistically significant if the p values were 0.05 or less.
Results
Hydrolysis by alcalase or papain under different pH and temperature conditions
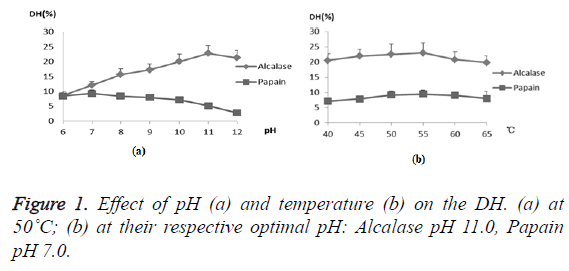
In order to investigate the feasibility of the compounding of alkaline protease and papain hydrolyzing proteins in one-step, the effect of the factors, pH and temperature, on hydrolysis by the two enzymes were examined, respectively. Usually, the extent of protein degradation by proteolytic enzymes was estimated by assessing the Degree of Hydrolysis (DH). Alkaline protease showed higher DH than papain under all temperature ranges and pH ranges except that at pH 6.0 (Figure 1). In addition, the DH of the two enzymes had similar tendencies when temperature ranged from 40˚C to 65˚C, and they both reached their respective optimums at 55˚C. However, in terms of pH, they reached their respective optimums at different pHs, with the alcalase at basic pH and papain at neutral pH. However, it is notable that the DH of CGM by papain retained about 90.9%, 85.9%, 76.9%, and 55.5% at pH 8.0, 9.0, 10.0, and 11.0 compared to that at optimum pH 7.0, showing a wide range of reaction pH values. Considering alkaline protease played major role in protein hydrolysis, it was feasible to apply the two enzymes in one-step. Hence pH 10.0 and 55˚C were chosen as center points with 0.5 and 5˚C as step changes, respectively.
Effect of other factors on hydrolysis through onefactor experimental design
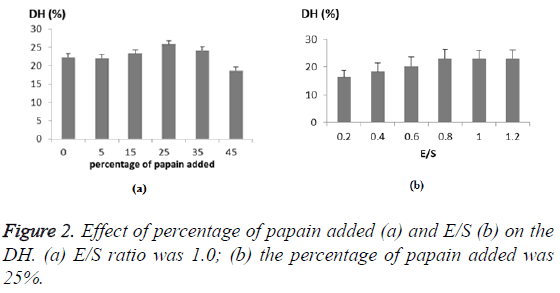
As the alcalase played a leading role in the hydrolysis, the mass percentage of papain was examined ranging from 0 to 45% (Figure 2a). The DH of CGM increased as the papain was added at increased amount, and reached the highest level of at 25 percent, which was 16% higher than with alcalase alone. Thus, the increased DH implied a cooperative effect on hydrolyzing protein between the two enzymes at one-step. Besides, the enzyme-substrate ratio (E/S, w/w) was examined, where the enzyme referred to the total mass of the two enzymes. As expected, the increase of E/S ratio resulted in an increase in DH of CGM due to greater hydrolysis of the protein (Figure 2b). Taking into account the DH achieved and the cost, the center point for E/S was 0.8 with a step change of 0.2 (Table 1). Hence, the center point for the percentage of the papain was 25% with a step change of 10% (Table 1).
Response surface method optimization
RSM was used to optimize the hydrolysis conditions for the preparation of the CPs from CGM. The influence of pH, temperature, E/S ratio and the percentage of added papain on the DH were shown in Table 2. Analysis of Variance (ANOVA) was presented in Table 3. As shown in Table 3, the value of p of the model was less than 0.05, indicating that the model was significantly predictive at the 0.05 significance level. The lack of fit analysis was used to test the fitness of the model. The pvalue of the lack of fit was 0.8075, which demonstrated that the lack of fit was not significant as compared to the pure error (p>0.05) which further validates the model. Thus, the model was able to fit the experimental data, and could be used to monitor the optimization. The following empirical regression Equation 3 represents the DH (y) on pH, temperature, E/S ratio, and the percentage of papain added.
| Source | Sum of squares | DF | Mean square | F value | p |
|---|---|---|---|---|---|
| Model | 47.92 | 14 | 3.42 | 2.60 | 0.0424* |
| X1 | 3.00 | 1 | 3.00 | 2.28 | 0.1536 |
| X2 | 0.2 | 1 | 0.20 | 0.15 | 0.7026 |
| X3 | 0.33 | 1 | 0.33 | 0.25 | 0.6228 |
| X4 | 0.38 | 1 | 0.38 | 0.29 | 0.6006 |
| X12 | 17.27 | 1 | 17.27 | 13.10 | 0.0028** |
| X22 | 7.47 | 1 | 7.47 | 5.67 | 0.0321* |
| X32 | 18.40 | 1 | 18.40 | 13.96 | 0.0022** |
| X42 | 3.85 | 1 | 3.85 | 2.92 | 0.1095 |
| X1 X2 | 3.50 | 1 | 3.5 | 2.65 | 0.1256 |
| X1 X3 | 0.23 | 1 | 0.23 | 0.17 | 0.6853 |
| X1X4 | 0.50 | 1 | 0.50 | 0.38 | 0.5490 |
| X2 X3 | 0.018 | 1 | 0.018 | 0.019 | 0.9081 |
| X2 X4 | 0.29 | 1 | 0.29 | 0.22 | 0.6453 |
| X3 X4 | 7.24 | 1 | 7.24 | 5.49 | 0.0344* |
| Statistical analysis | |||||
| Residual | 18.45 | 14 | 1.32 | ||
| Lack of fit | 10.56 | 10 | 1.06 | 0.53 | 0.8075 |
| Pure error | 7.89 | 4 | 1.97 | ||
| Cor total | 66.37 | 28 | |||
Table 3: Statistical analysis for the response surface quadratic model obtained from RSM design.
The model had an acceptable coefficient of determination (R2=0.8524), which indicated that 85% of the variability in the response on DH can be explained by the independent parameters. The term “Predicted R-Squared” agreed well with the “Adjusted R-squared” (data not shown). These results indicated that the models were well adapted to the responses.
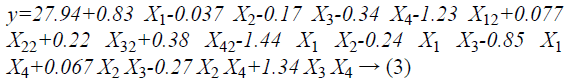
Where y, X1, X2, X3, X4 were the DH, pH, temperature, E/S ratio and the percentage papain added, respectively.
Effects of parameters
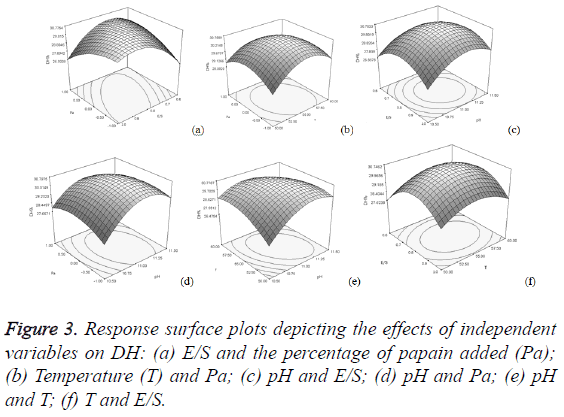
In order to determine the optimal levels of each variable for maximum DH, 3D response surface plots were constructed by plotting the DH on the Z-axis against any two independent variables, while maintaining other variables at their optimal levels (Figure 3). The DH increased until pH, temperature, E/S ratio and the percentage of papain added reached an optimum point and then declined following further increasing of the above variables’. This demonstrated that those variables could affect the enzyme hydrolysis activity, and there should be an optimal value of DH in the range. A relative alkaline pH and high E/S were desirable to promote the DH and the fluctuation around the center point in temperature and the percentage of papain added did not result in improved DH. Factorial effects with values of p less than 0.05 were considered to be significant. It was surprising that not a single factor exerted a significant linear effect of DH within a 95% confidence interval, while the quadratic effects of three factors, including pH (X1) (p<0.01), temperature (X2) (p<0.05), and the E/S ratio (X3) (p<0.01), were significant (Table 3). In addition, the interaction effect between E/S ratio and the percentage of papain added (X3 X4) were significant (p<0.05). Hence, in our study, the four variables exhibited complicated effects on the hydrolysis reaction.
Optimization and model validation
The optimal conditions were extracted by Minitab 14 software. The highest DH obtained by using the above optimized concentrations of the variations was pH at 11.10, temperature at 55˚C, E/S ratio at 0.85, and percentage of papain added at 20%. On this condition, the predicted DH was 30.84%. To confirm the validity of the model, hydrolysis of CGM was performed under the optimal conditions, and the DH reached 29.51 ± 2.49%. The experimental DH value agreed with value predicted by the model within a 95% confidence interval, and it was about 20% higher than the value obtained through OFAT. This confirmed that the model was powerful and suitable for the estimation of experimental values.
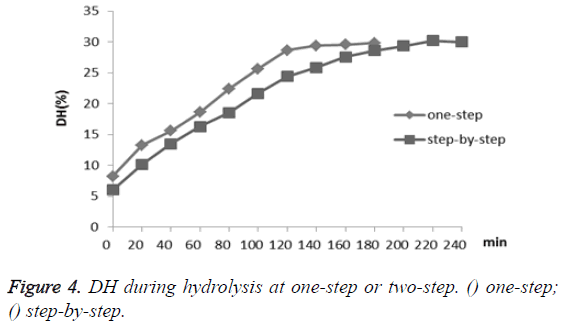
Besides, the DH of one-step hydrolysis was examined every twenty minutes in three hours, and the step-by-step hydrolysis was also conducted using the alkaline protease for two hours and then papain for another two hours under their optimum conditions, respectively (Figure 4). The DH of CGM by onestep hydrolysis increased rapidly during the first two hours, and then DH level remained at about 29%; while the step-bystep hydrolysis reached a higher DH at 30% compared to the one-step after hydrolysing for four hours. Given the fact that similar DH level can be reached with much less effort and time, one-step hydrolysis clearly has advantage over the traditional step-by-step approach in terms of efficiency.
Effects on the exhaustive swimming
Loaded swimming exercise of mice was selected for evaluation of the CPs prepared from the CGM on anti-fatigue activity. The length of the exhaustive swimming time, BLA and BUN content in blood, and liver glycogen and muscle glycogen contents of mice were examined to indicate the degree of fatigue or the state related to fatigue. As shown in Table 4, the exhaustive swimming times of the Low, Med, and High groups were longer than that of the control group (p<0.05), and increased by 27.69%, 106.16%, and 138.47%, respectively. The liver glycogen and muscle glycogen contents of mice in the Med and High groups were significantly increased compared with that in the control group (p<0.05) (Table 4). The contents of glycogen in the liver and muscle in the Low group were also raised by 10.01% and 35.77%, respectively, though no significant difference was observed (p>0.05) (Table 4). The effects of the CPs on BLA and BUN of mice after swimming were also depicted (Table 4). Though the BLA was decreased in all three experiment groups compared with that in the control group, only the BLA in High groups exhibited significant difference (p<0.05) (Table 4). The BUN contents of mice in all the experimental groups were higher than that in the control, though only that in High group had a significant difference compared with that in the control group (p<0.05).
| Group | Swimming time (min) | Liver glycogen (mg/g) | Muscle glycogen (mg/g) | BUN (mmol/L) | BLA (mmol/L) |
|---|---|---|---|---|---|
| Control | 43.33 ± 6.73 | 6.99 ± 1.33 | 0.99 ± 0.11 | 29.44 ± 6.16 | 13.54 ± 0.93 |
| Low | 55.33 ± 4.50* | 7.69 ± 1.92 | 1.61 ± 0.50 | 29.28 ± 0.21 | 13.37 ± 0.49 |
| Med | 89.33 ± 5.25* | 9.49 ± 3.70* | 2.16 ± 1.19* | 31.67 ± 6.00 | 12.19 ± 0.23 |
| High | 103.33 ± 9.50* | 14.08 ± 7.182* | 2.76 ± 1.53* | 38.37 ± 1.22* | 11.45 ± 1.76* |
Table 4: Comparisons of swimming time, liver glycogen, muscle glycogen, contents of BLA and BUN (n=10).
Discussion
To obtain the high hydrolysis degree of the CGM as soon as possible in proper conditions, the feasibility of alkaline protease and papain under the uniform condition was investigated. Alkaline protease (E.C. 3.4.21.14) from Bacillus sp. is characterized by its optimum activity and stability under alkaline condition. Acting as an endopeptidase, they are known to be specific against aromatic or hydrophobic amino acid residues such as phenylalanine, tyrosine, or leucine at the carboxyl side of the splitting point [21]. While papain (E.C. 3.4.22.2), still an endopeptidase, is characterized by a remarkable preference for hydrophobic amino acids at the P2 site of the scissile peptide bonds [22]. If some favourable conditions were met, the two proteases might work together and provide synergistic effects on the proteolysis of CGM. In our study, though the papain’s optimum of pH is about 7.0, the DH of CGM by papain around pH 11.0 still retained above half peak values. As the optimum pH of papain is known to be neutral, at pH 6.5 to 7.0, there is little information about the digestion of protein by papain under alkaline condition. However, it is well known that the rate of digestion at a certain pH also varies with the different substrates, that is, the amino acids of the peptide and their arrangement in the chain. Lineweaver and Schmimmer found that the activity of papain at pH 9.0 was about 60 percent of that at pH 6.5 to 7.0 [23]. Hoover investigated the effect of pH on the course of proteolysis by papain using three synthetic substrates, Benzoylargininamide (BAA), Carbobenzoxy-l (+)- Isoglutamine (CBIG), and Hippurylamide (HA) [24]. The result exhibited that the optimum pH for the BAA is 0.5 units higher than those for the other two substrates, and HA, the least readily digestible substrate, had a slightly broader curve than the others. In our study, the degree of hydrolysis of CGM by papain was 9.21% even at its optimum pH 7.0, a quite lower level compared with that of about 17% of lotus seed protein in optimal conditions [25]. It is uncertain whether the wide range of pH for the proteolysis of CGM was related to the low digestible substrate of CGM. Anyway, it provided an opportunity for the combined use of the two proteases under alkaline condition.
It is well documented that a divalent cation is required by alkaline protease not only for its maximum activity, but also for the increase of its thermal stability [26]. So the effect of metal ions, including Mg2+, Mn2+, Ca2+, Cu2+, K+, and Zn2+, on the DH of alkaline protease along was studied at the concentrations ranging from 2 mmol to 10 mmol. However, in this study, there was no significant enhancement of DH when applying each kind of metal ion at different concentrations compared to the blank control (p>0.05), though supplementation with 4 mmol Mn2+ showed a slight increase on DH by 7% (p>0.05) (Data not shown). A severe inhibition of DH by Cu2+ (≥4 mmol) was observed in the hydrolysis system (p<0.05). The effect of cations on alkaline proteases varied with different proteinases derived from different microorganism [27], and that might yet be interrelated with different substrates.
Experiments were carried out to obtain the optimal enzymatic hydrolysis condition. Based on the OFAT, three different levels for per factor were chosen respectively in the following study. The purpose of the ANOVA was to investigate whether the model proved effective and which parameter significantly affected the response [28]. Usually, the small p-value indicated that the model or the factor made a significant effect. The results of ANOVA for DH showed that the model was accepted as significant at the level of 0.05 confidence level. While none of the four factors was linearly significant at a significance level of 0.05, and the quadratic effects of three factors, including pH, temperature, and the E/S ratio were accepted as significant at the level of 0.05. The result was unusual, for in the majority of studies there were usually some of the parameters making significant effects on the response [9,20,29]. This might be related partly to the DH of CGM hydrolyzed by complex alkalase-papain system, in which the factors might influence the two proteases in different ways, thus making them become more complicated to the response.
After the hydrolysis period, the hydrolysate was mixed up with activated charcoals for the absorption of AAAs (data not shown), and then it was fractioned using ultrafiltration to obtain the fraction of oligopeptides with mass molecules under 3 KDa. The permeate turned out to have a molecular weight distribution ranging from 109 Da to 624 Da by separation of Sephadex G-15, with a relatively rich content of BCAAs (a molar percent of 24.3 to total amino acids), and a relatively low content of AAAs (a molar percent of 1.1 to total amino acids) (unpublished data). Having the molar ratio of BCAAs/ AAAs to 21.92, the mixture obtained can be considered the CPs with high Fischer’s ratio (defined as the ratio higher than 20.0) [30]. As the mixture of BCAAs supplementation has been demonstrated to enhance glycogen reserves in trained rats either in rested state [31] or in moderate exercise [32] and enhance mitochondrial biogenesis, we further investigated the anti-fatigue activities of the CPs prepared from CGM.
Loaded swimming exercise of mice was used to evaluate the anti-fatigue activity of the CPs [33]. In our study, the data showed that the CPs significantly prolonged exhaustive swimming time of mice in the Med or High groups compared to that in the control (p<0.05), which suggested that the CPs with a high content of BCAAs had anti-fatigue activity. This agrees with the result of human test, when healthy individuals supplemented with mixture of BCAAs (300 mg/kg/d) for 3d demonstrated a greater resistance to fatigue and higher plasma glucose during an exhaustive exercise test [34]. However, Campos-Ferraz argued that Leucine (Leu) alone could prevent impaired endurance exercise performance compared with the mixture of BCAAs [35]. In addition, other studies indicated that supplementation of some amino acids, esp. Leu, preserves liver glycogen, which is fundamental to glucose homeostasis and thus resistance to exhaustion [36-38]. It was worth noting that the content of Leu was relatively high in the CPs prepared from the CGM, accounting for 14.7% of the total amino acids (data not shown), thus the CPs with relatively high contents of either BCAAs or Leu might act as agents for anti-fatigue. The potential mechanism was described as increased serum Leu could inhibit Branched-Chain Keto acids Dehydrogenase complex (BCKD) and hence stimulate BCKD dephosphorylation (activation), increasing the oxidation of other essential amino acids, such as isoleucine and valine, which contributed indirectly to muscle and liver glycogen sparing [35]. As the glycogen is the important resource of energy during exercise, the increase in glycogen stored in liver or muscle will improve the body’s physical endurance [39]. That was in accordance with our result that the CPs significantly increased muscle and liver glycogen contents of mice after exhaustive swimming. Besides, increased Leu implied in a lower pyruvate dehydrogenase activity in muscle and an augmented concentration of alanine, which goes to the bloodstream and reaches the liver, making it possible to generate more glucose through the glucose-alanine cycle [35]. Therefore supplementation with Leu could result in an increased glycogen store as well as stronger amino acid metabolism. That is consistent with our data that the BUN contents of mice in the experiment groups rose along with the increasing dosage of the CPs compared to that in the control group. BUN, the metabolic outcome of protein and amino acid, acts as another index to evaluate the bearing capability when the body suffers from a physical load. Usually, there is a negative correlation between the urea nitrogen in vivo and the exercise tolerance, because protein and amino acids have a stronger ketabolic metabolism when the body is unable to obtain enough energy by sugar and fat catabolic metabolism [40]. But in our study, the enhanced BUN content of mice in the experiment groups may be due to the activity of the Leu and/or the additional supplement of the CPs. Therefore, BUN might not be a proper index for the physical fatigue when the supplementation is involved in the proteins, oligopeptides, or amino acids. BLA is also an important biochemical parameter related to fatigue. As glycolysis is the main energy source for intense exercise in a short time, BLA, the glycolysis product of carbohydrate under an anaerobic condition, could act as an important indicator for judging the degree of fatigue [41]. Our results consistently showed that the CPs could inhibit the increase of BLA of mice after swimming, which suggested that the CPs could postpone the appearance of fatigue.
Conclusion
In conclusion, our results indicated that CGM could be effectively hydrolyzed using the combined papain and alkaline protease to obtain the high Fisher-value CPs with an antifatigue activity. RSM was an efficient statistical tool in the optimization of the hydrolysis conditions. The highest DH was 29.51%, at a reaction temperature of 55˚C, a reaction pH at 11.10, an E/S ratio of 0.85, and the percentage of papain added at 20%. The experimental value agreed with the predicted value within a 95% confidence interval, suggesting a good fit between the models and the experimental data. The CPs prepared from the hydrolysates could be characterized by high Fisher-values, rich in BCAAs and Leu, and exhibited an effective anti-fatigue effect. The data showed that the CPs could prolong the exhaustive swimming time of mice, as well as increase liver and muscle glycogen contents and decrease BLA contents. The results supported that the CPs had an antiphysical fatigue effect. Future study could be directed towards the purification and generation of bioactive peptide sequences to better understand the mechanism involved in the action of the CPs on physical fatigue.
Acknowledgements
This work was financially supported by the Bureau of Science and Technology of Jinan Municipality (TNK0908) and the Department of Education of Shandong Province (J12LF10).
References
- Ortenblad N, Westerblad H, Nielsen J. Muscle glycogen stores and fatigue. J Physiol 2013; 591: 4405-4413.
- Kruegera GP. Sustained work, fatigue, sleep loss and performance: A review of the issues. Work Stress 1989; 3: 129-141.
- Bassett DR, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sport Exerc 2000; 32: 70-84.
- Fu X, Ji R, Dam J. Antifatigue effect of coenzyme Q10 in mice. J Med Food 2010; 13: 211-215.
- Wang X, Lei L, Zhang J. Antioxidant and anti-fatigue activities of flavonoids from Puerariae Radix. Afr J TradCompl Alt Med 2012; 2: 221-227.
- Shan-shan L, Zi-chao C, Chao-hui ZPE. Effect of Tao-Hong-Si-Wu-Tang, a traditional Chinese herbal medicine formula, on physical fatigue in mice. Afr J TradCompl Alt Med 2013; 10: 60-65.
- Patricia LCF, Thomas B, Humberto N, Antonio HL. Distinct effects of leucine or a mixture of the branched-chain amino acids (leucine, isoleucine, and valine) supplementation on resistance to fatigue, and muscle and liver-glycogen degradation, in trained rats. Nutrition 2013; 29: 1388-1394.
- Saibo H, Huimin L, Shang-gui D. Study of anti-fatigue effect in rats of ferrous chelates including hairtail protein hydrolysates. Nutr 2015; 7: 9860-9871.
- Zhuang, Tang N, Dong ST, Sun B, Liu JB. Optimisation of antioxidant peptide preparation from corn gluten meal. J Sci Food Agric 2013; 93: 3264-3270.
- Lin F, Chen L, Liang R, Zhang Z, Wang J, Cai M, Li Y. Pilot-scale production of low molecular weight peptides from corn wet milling byproducts and the antihypertensive effects in vivo and in vitro. Food Chem 2011; 124: 801-807.
- Li HM, Hu X, Guo P, Fu P, Xu LI, Zhang XZ. Antioxidant properties and possible mode of action of corn protein peptides and zein peptides. J Food Biochem 2010; 34: 44-60.
- Miao FS, Yu WQ, Wang YG, Wang MJ, Liu XY, Li FL. Effects of corn peptides on exercise tolerance, free radical metabolism in liver and serum glutamic-pyruvic transaminase activity of mice. Afr J Pharm Pharmacol 2010; 4: 178-183.
- Li XX, Han LJ, Chen LJ. In vitro antioxidant activity of protein hydrolysates prepared from corn gluten meal. J Sci Food Agr 2008; 88: 1660-1666.
- Guo H, Sun J, He H, Yu GC, Du J. Antihepatotoxic effect of corn peptides against Bacillus Calmette-Guerin/lipopolysaccharide-induced liver injury in mice. FoodChemToxicolInt J Publ Br IndBiol Res Assoc 2009; 47: 2431-2435.
- Ma ZL, Zhang WJ, Yu GC, He H, Zhang Y. The primary structure identification of a corn peptide facilitating alcohol metabolism by HPLC-MS/MS. Peptides 2012; 37:138-143.
- Alessandra V, Giuseppe D, Enzo N. Branched-chain amino acids, mitochondrial biogenesis, and healthspan: an evolutionary perspective. Aging 2011; 3: 464-478.
- Sami S, Nazamid S, Farooq A, Azizah AH, Hasanah MG. Recent advances in food biopeptides: Production, biological functionalities and therapeutic applications. BiotechnolAdv 2015; 33: 80-116.
- Corradini E, Curti PS, Meniqueti AB, Martins AF, Rubira AF, Muniz EC. Recent advances in food-packing, pharmaceutical and biomedical applications of zein andzein-based materials. Int J MolSci 2014; 15: 22438-22470.
- Adler-Nissen J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J Agric Food Chem 1979; 27: 1256-1262.
- Cornelly V, Harry G, Dries BA, Alphons GJ. Optimisation of the angiotensin converting enzyme inhibition by whey protein hydrolysates using response surface methodology. Int Dairy J 2002; 12: 813-820.
- Morihara K. Comparative specificity of microbial proteinases. AdvEnzymolRelat Areas MolBiol 1974; 41: 179-243.
- Hamid M, Ikramul H. Comparative evaluation of agroindustrialbyproducts for the production of alkaline protease by wild and mutant strains of Bacillus subtilis in submerged and solid state fermentation. Sci World J 2013; 2013: 538062-538067.
- Lineweaver H, Schwimmer S. Effect of urea on trypsin and alpha-chymotrypsin. Enzymologia 1941; 10: 81.
- Hoover SR, KokesEL. Effect of pH upon proteolysis by papain. J BiolChem 1947; 167: 199-207.
- Juan M. The effect of Hg2+ on papain catalytic performations and its mechanism. Master degree, Xiangtan University, Xiangtan, China 2011.
- Paliwal N, Singh SP, Garg SK.Cation-induced thermal stability of an alkaline protease from a Bacillus sp.BioresourceTechnol 1994; 50: 209-211.
- Ganesh KC, Hiroshi T. Microbial alkaline proteases: From a bioindustrial viewpoint. BiotechnolAdv 1999; 17: 561-594.
- Chiang KT, Chang FP, Tsai TC. Optimum design parameters of Pin-Fin heat sink using the grey-fuzzy logic based on the orthogonal arrays. Int JHeat Mass Transf 2006; 33:744-752.
- Xiaoqing R, Lizhen M, Ju C, Yong HW, Ying PZ, Siliang Z, Hong SY,Hongjie C. An optimization of enzymatic hydrolysis of channel catfish bones for preparingantimicrobial agents. JAquat Food Prod Technol 2012; 21: 99-110.
- Fischer JE, Yoshimura N, Aguirre A, James JH, Cummmg MG, Abel RM, Deindoerfer F. Plasma amino acids in patients with hepatic encephalopathy. Effects of amino acid infusions. Am J Surg 1974; 127: 40-70.
- Campos-Ferraz PL, Ribeiro SML, Luz SD, Lancha AH, Tirapegui J. Exercise BCAA supplementation in young trained rats: what are their effects on body growth? J SportSci Med 2011; 10: 483-490.
Falavigna G, Alves de Araujo J, Rogero MM, Pires IS, Pedrosa RG, Martins E. Effects of diets supplemented with branched-chain amino acids on the performance and fatigue mechanisms of rats submitted to prolonged physical exercise. Nutrients 2012; 4:1767-1780. - An HJ, Choi HM, Park HS, Han JG, Lee EH, Park YS, Um JY, Hong SH, Kim HM. Oral administration of hot water extracts of Chlorella vulgaris increases physical stamina in mice. Int J Immunopharmacol 2006; 50: 380-386.
- Gualano AB, Bozza T, Lopes De Campos P, Roschel H, Dos Santos Costa A,LuizMarquezi M. Branched-chain amino acids supplementation enhances exercise capacity and lipid oxidation during endurance exercise after muscle glycogen depletion. J Sport Med Phy Fitness 2011; 51: 82-88.
- Campos-Ferraz PL,Bozza T,Nicastro H,Lancha AH. Distinct effects of leucine or a mixture of the branched-chain amino acids (leucine, isoleucine, and valine) supplementation on resistance to fatigue, and muscle and liver-glycogen degradation, in trained rats. Nutrition2013; 29: 1388-1394.
- Donato J, Pedrosa RG, Cruzat VF, Pires IS, Tirapegui J. Effects of leucine supplementation on the body composition and protein status of rats submitted to food restriction. Nutrition 2006; 22: 520-527.
- Falavigna G, Alves de Araujo J, Rogero MM, Pires IS, Pedrosa RG, Martins E. Effects of diets supplemented with branched-chain amino acids on the performance and fatigue mechanisms of rats submitted to prolonged physical exercise. Nutrients 2012; 4:1767-1780.
- Marquezi ML, Roschel HA, dos Santa Costa A, Sawada LA, Lancha AH. Effect of aspartate and asparagine supplementation on fatigue determinants in intense exercise. Int J Sport NutrExercMetabol 2003; 13: 65-75.
- Madsen K, Pedersen PK, Rose P, Richter EA. Carbohydrate supercompensation and muscle glycogen utilization during exhaustive running in highly trained athletes. Eur JApplPhysiolOccupPhysiol 1990; 61: 467-472.
- Wei W, Zheng LY, Yu MY, Jiang N, Yang ZR, Luo X. Anti-fatigue activity of extract form the submerged fermentation of Ganodermalucidum using Radix astragali as substrate. J Animal Plant Sci 2010; 6: 677-684.
- Yu B, Lu ZX, Bie XM, Lu FX, Huang XQ. Scavenging and antifatigue activity of fermented defatted soybean peptides. Eur Food Res Technol 2008; 226: 415-421.