ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2009) Volume 20, Issue 1
Prevalence of Methicillin-Resistant Staphylococcus aureus in a teaching hospital in Riyadh, Saudi Arabia
Awadh R. Al-Anazi*
Infectious Diseases Division, Department of Medicine (38), King Khalid University Hospital, College of Medicine, King Saud University, Riyadh, Saudi Arabia
- *Corresponding Author:
- Awadh R. Al-Anazi
Infectious Diseases Division
Department of Medicine (38)
King Khalid University Hospital
College of Medicine, King Saud University
P.O. Box 2925, Riyadh 11461
Saudi Arabia
E-mail: ara2328@gmail.com, awadh@ksu.edu.sa
Accepted date: October 23 2008
Following the first report in 1961, methicillin–resistant Staphylococcus aureus (MRSA) has progressively become a leading cause of nosocomial infections. MRSA infection may have a significant impact on patient morbidity and mortality. Limited number of studies in Saudi Arabia has attempted to investigate infection and risk factors associated with nosocomial acquired MRSA. This study was designed to estimate the hidden prevalence of MRSA at King Khalid Uni-versity Hospital (KKUH), Riyadh, Saudi Arabia and identified and described the potential risk factors for MRSA infection. The study was carried out at KKUH over a period of 12 months. All the cases of hospital acquired MRSA were included. The demographic, clinical and laboratory data of the in-fected patients were obtained. Isolation and susceptibility of MRSA were performed at KKUH microbiology laboratory. The risk factors for colonization were estimated. Informa-tion concerning hospitalization, antibiotic treatment in the previous two months, and the presence of co-morbidities were obtained. The chart of patients were reviewed daily for in-dication and duration of antimicrobial therapy. The date of discharge and the duration of hospital stay were calculated. This study revealed an MRSA prevalence of 2 cases per 1000 admission. Diabetes mellitus, multiple co-morbidities, old age, male gender, antibiotics use and I.C.U. admission were sig-nificant risk factors associated with increased prevalence of MRSA acquisition. The presently applied infection control policies at KKUH were effective in controlling MRSA infection. However more studies are suggested to further explore means of further reducing MRSA burden on health systems.
Keywords
Methicillin-resistant, nosocomial infection, demographic, diabetes mellitus
Introduction
Staphylococcus aureus, often referred to simply as “staph”, are commonly carried on the skin or in the nose of healthy people. Occasionally, S. aureus can cause skin infections and most of the times these infections are mi-nor (such as pimples and boils) and can be treated without antibiotics. However, it can also cause serious infections such as surgical wound infections and pneumonia, espe-cially in children and immunocompromised patients [1]. In the past, most of the serious S. aureus infections were treated with penicillin. However, over the past 50 years, treatment of these infections has become more difficult because of the development of resistance in S. aureus to various antibiotics, including the commonly used penicil-lin-related antibiotics [2]. The term “methicillin-resistant Staphylococcus aureus” (MRSA) refers to those strains of S. aureus that have acquired resistance, whether in the community or in the hospital, to the antibiotics methicil-lin, oxacillin, nafcillin, cephalosporins, Imipenem, and/or other beta-lactam antibiotics [1,2]. Primarily, MRSA used to be a nosocomial pathogen that is prevalent in most hospitals and endemic at some institutions [1,3]. MRSA is now considered a virulent organism that can cause significant mortality and morbidity, and although it does not seem to affect healthy individuals, it can be deadly to patients in hospitals [3,4]. The main reason for the increased risk of mortality to patients acquiring MRSA in critical care areas (CCAs) is that these patients are already compromised by other disease processes and are also often immunosuppressed [5,6]. Therefore, con-siderable emphasis needs to be placed on identifying ef-fective procedures to control the spread of MRSA within hospitals. Studies have shown that, once introduced into a hospital, MRSA is difficult to eradicate, with fewer than 15% of all hospitals that reported outbreaks able to elimi-nate the organism [7].
Methicillin resistance in Staphylococcus aureus was first reported in 1961 and since then MRSA has gone from being regarded as “not only rare but of doubtful clinical significance” to a major nosocomial pathogen throughout the world [1,3,8,9]. Although Jevons [8] was the first to report MRSA shortly after the introduction of methicillin in 1959, MRSA did not become a problem until the late 1970s when it emerged as a major cause of staphylococ-cal infections in hospitals worldwide [1,3,6,8,9].
MRSA is a staphylococcal that is resistant to methicillin. This implies resistance to all penicillinase-resistant penicillins and essentially, all other ß-lactam antibiotics. In the late 1960s and 1970s, MRSA has also been found to be resistant to other unrelated antibiotics, such as strep-tomycin, tetracyclines, Chloramphenicol, erythromycin, lincomycin, clindamycin and kanamycin [10]. Therefore, MRSA infections have the serious clinical implications, in that they are difficult to treat and are associated with increased hospital stay and/or death [4,11,12]. Duckworth et al [4] reported a higher incidence of septic shock, bac-teraemia and three times greater risk of death in patients with MRSA in comparision to patients with Methicillin sensitive Staphylococcus aureus (MSSA) [4]. Initially, MRSA was thought to be less pathogenic but further find-ings in both Australia and the USA found MRSA was capable of producing serious diseases such as empyema, pneumonia, osteomyelitis, enterocolitis and septicaemia [10]. The significance for patients with an MRSA infec-tion is that their treatment is now limited to the use of a narrow range of potentially toxic antibiotics such as van-comycin and Teicoplanin [4]. Vancomycin-resistant Staphylococcus aureus (VRSA) and vancomycin-resistant enterococci (VRE) are now major problems in both hospi-tal and community health care facilities worldwide [13,14].
The development of resistance to these antibiotics is growing at such an alarming rate that current hospital inf-ection control measures may prove ineffective. Since de-laying initiation of effective MRSA therapy has been shown to be a significant mortality risk factor, research has begun to focus on developing new antibiotics to manage infections caused by gram positive bacteria, such as MRSA [4]. Linezolid and the combination quinupristin / dalfopristin (Synercid™) are the first of two new series of antibiotics being introduced to help combat the problems of resistance. Currently, these antibiotics are mainly used in the treatment of serious infections in CCAs and immu-nompromised patients where resistance to first line anti-biotics has been demonstrated [13]. However, to preserve the long-term value of these antibiotics, hospitals must ensure that clear guidelines and policies are in place for the prescribing and use of these new drugs. Once MRSA is established, it often spreads rapidly among the severely ill. Modes of transmission include transient contamina-tion from patient to patient via the unwashed hands of medical staff and nurses, and contact spread from health care workers either asymptomatically colonized in the anterior nares or on their hands [10]. Other modes of transmission are contact with heavily contaminated linen, equipment and environmental surfaces around infected patients. Instruments and equipment (e.g. stethoscopes, writing equipment, ventilators, lifting slings, sphygma-nometers) should be identified and designated for MRSA patients only, and preferable for individuals. If this is not possible and to prevent possible cross contamination, dis-infection with an appropriate solution (70% alcohol) should be carried out before using these items on other patients [4]. Patients with MRSA pneumonia or those receiving mechanical ventilation can cause the droplet spread of MRSA during coughing or physiotherapy.
In Saudi Arabia, very few studies have been conducted so far to investigate the incidence or prevalence of MRSA and identification of the risk factors associated with MRSA infection in the community and/or hospital pa-tients [15-26]. The first report came in the international literature in the year 1992 by Al-Masudi et al, in which a comparative study to investigate the sensitivity of MRSA strains isolated from Saudi Arabia and great Britain to antibiotics and biocides was undertaken [15]. They have reported that Saudi and British strains differed in their sensitivity pattern, as none of the Saudi strains showed resistance to propamidine isethionate. Whereas, most of the British Gentamicin-resistant MRSA have been highly resistant to this agent and other nucleic acid binding com-pounds [15]. In 1993, Haddad et al, documented the first outbreak of MRSA in neonatal intensive care unit at a tertiary care hospital in Riyadh [16]. They reported that all of the MRSA were resistant to gentamicin, erythromy-cin, tetracycline but sensitive to vancomycin [16] Over-crowding, limited space, and inadequate cleaning were incriminated as the major risk factors [16].
First epidemiological report on MRSA from Saudi Arabia appeared in the year 1994 from the western region (Jeddah) [17]. MRSA comprised about 7.5% of all S. aureus isolates studied over a period of 3 years, with 71% of them originating from wound sites [17]
Majority of them were resistant to tetracycline (93%), followed by gentamicin (83%), rifampicin (6%) and cipr-ofloxacin (1%) [17]. Alghaithy et al have investigated the carrier status and antibiotic resistance among hospital and non-hospital personnel in Abha (Saudi Arabia) and isolated MRSA from 5.1% of the hospital and 18.3% from the non-hospital carriers [19]. However, 44% of the hospital carriers were multiple resistant as compared to 8% of the non-hospital carriers. Moreover, multiple resistance was more common among in-patients (8%) as compared to out-patients (1%) [17]. Madani et al and several other authors [19-25] have shown that prevalence of MRSA at tertiary care hospitals is increasing in the kingdom, and therefore control measures are needed to be enhanced and effectively applied.
Literature survey has shown that no study has been conducted so far to estimate the prevalence of MRSA at KKUH one of the biggest tertiary care health centers in the Kingdom of Saudi Arabia. The objective of this prospective study is to determine the prevalence of MRSA at KKUH and also describe the potential risk factors.
Patients and Methods
a. Patients
This prospective study was carried at the King Khalid University Hospital (KKUH), (a 680 beds tertiary-care hospital located in Saudi Arabia, Capital City-Riyadh, of these beds, 62 are intensive care beds, 28 adults and 34 pediatrics), over a period of 12 months from 01 August 2005 to 31July 2006. Demographic, clinical and labora-tory data were prospectively collected, through a struc-tured questionnaire, on all hospital acquired (HA)-MRSA cases, regardless of age, sex, and nationality. MRSA was defined as reported by the microbiology laboratory. Cases were classified as infection according to center of disease control and prevention (CDC) criteria [18]. Cases associated with no clinical infection were considered colonized. Only nosocomially acquired MRSA were included.
b. Clinical definitions
Nosocomial or HA-MRSA was defined as the MRSA acquired after 72-hours of hospitalization or related to an intervention during the hospital stay [23].
The sites of infection: Skin and Soft tissue infection, sur-gical site infection, Blood stream infection, Urinary tract infection, pneumonia and catheter related infections were defined according to CDC guidelines [18].
Empiric therapy was defined as treatment of a presumed infection for which no pathogen or source could be de-termined; therapeutic antimicrobial use was defined as treatment of a microbiologically or clinically documented infection; and prophylactic therapy was defined as treat-ment given to prevent infections in patients undergoing surgical and invasive procedures.
c. Data collection
After obtaining the informed consent of the participants, swabs were obtained from the anterior nares, baseline demographic variables, clinical and laboratory data were recorded through the completion of a well structured questionnaire (Appendix ) at the patient’s bedside. Data of patients found to be newly colonized with MRSA, were collected. Risk factors were extracted from the medical records. These included patient’s underlying conditions, frequency of previous medical consultations, the reason for current admission, history of previous hospital admissions, transferals between wards, antibiot-ics used, the intensity of the care required during the on-going episode of admission and the presence of chronic skin ulcers. Repeat and duplicate isolates from the same person during the study period were identified by the pa-tient medical record number and excluded. The risk fac-tors for colonization were estimated by interview and daily chart review. Information was obtained concerning hospitalization in the preceding year, whether the patient was admitted from the community, another hospital or transferred from another ward of the same hospital, anti-biotic treatment in the previous 2 months, and the presence of diabetes. The charts of patients were reviewed daily for antibiotic use, including the indication for, and the duration of anti-microbial therapy. The dates of dis-charge were noted and the duration of hospital stay calculated.
d. Microbiological investigations:
Initial screening and identification of S. aureus were ac-cording to standard laboratory protocols. Briefly, speci-mens were streaked on mannitol salt agar and isolates of S. aureus were tested for methicillin resistance using a standard oxacillin salt agar screening plate procedure and Cefoxitin susceptibility as indicated by the National Committee for Clinical Laboratory Standards (NCCLS). A methicillin-resistant strain and a methicillin-sensitive strain were included as controls. Isolation of even one colony of S. aureus on the selective medium was consid-ered indicative of MRSA.
e. Screening and confirmation of methicillin-resistance
Screening of methicillin-resistance were done by inoculat-ing a 10 μL aliquot of a bacterial suspension with 3 X 106 CFU/ml (McFarland turbidity no. 1) on Mueller- Hinton agar supplement with Ca (50 mg/L), Mg (25 mg/L), NaCl (4%) and oxacillin (4 μg/ml). A growth of at least one well-defined colony on these plates incubated at 30°C for 48h will be suggestive of MRSA.
The methicillin-resistance strain were confirmed by mi-crodilution in Mueller-Hinton broth supplemented with cations and NaCl, using a final inoculation of 7.5 x 104 CFU. MRSA was identified with a minimal inhibitory concentration (MIC) of oxacillin >8 μg/ml, and those with an MIC <1μg/ml will be considered MSS. An MIC of oxacillin between >1μg/ml and <8 μg/ml were defined a methicillin-intermediate Staphylococcus (MIS). This was confirmed by cefoxitin susceptibility following ClSi recommendation. MICs for vancomycin, gentamicin, ery-thromycin, penicillin, tetracycline, clindamycin, ami-kacin, ciprofloxacin and trimethoprim-sulfamethoxazole were carried out according to the recommendations of NCCLS using S. aureus ATCC 29212 as control.
Results
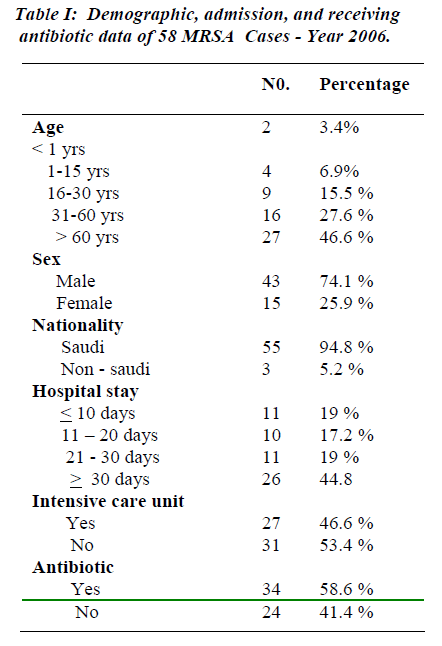
During the period of study 27,655 adult patients were admitted to KKUH and 58 patients developed 68 episodes of MRSA infections. This gives prevalence of 2 cases per 1000 admissions. Twenty-seven patients (46.6%) were above 60 years of age; 16 patients (27.6%) ranged in their age between 31 and 60 years, while 9 patients (15.5%) were at 16-30 years age groups. Lowest MRSA infection occurred at the age less than one year (2 patients, i.e. 3.4%) while 4 patients (6.9%) were in the age range of 1-15 years. Forty three patients (74.1%) were males and 15 were females (25.9%). The majority of patients were Saudi (94.8%). Eleven patients had a hospital stay of < 10 days (19%), while the hospital stay of 11-20 days and 21-30 days was spent by 10 patients (17.2%) and 11 pa-tients (19%) respectively. Twenty six patients (44.8%) stayed for > 30 days. Twenty-seven patients (46.6%) re-quired admission to intensive care units (I.C.U.) while 31 patients (53.4%) were admitted to general wards. Thirty-four patients (58.6%) received antibiotic treatment for MRSA infection and 24 patients (41.4%) received no an-tibiotics as they were only colonized, rather than, infected with MRSA.
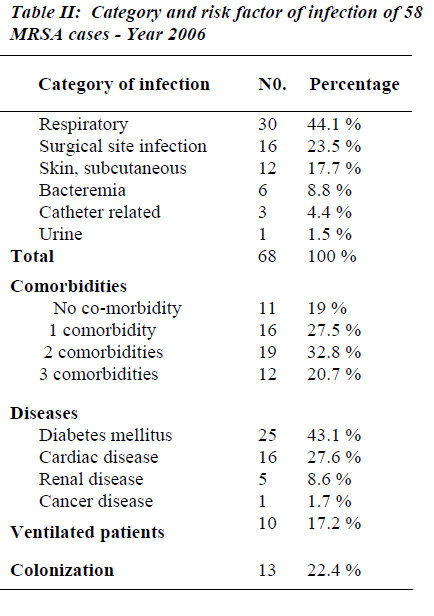
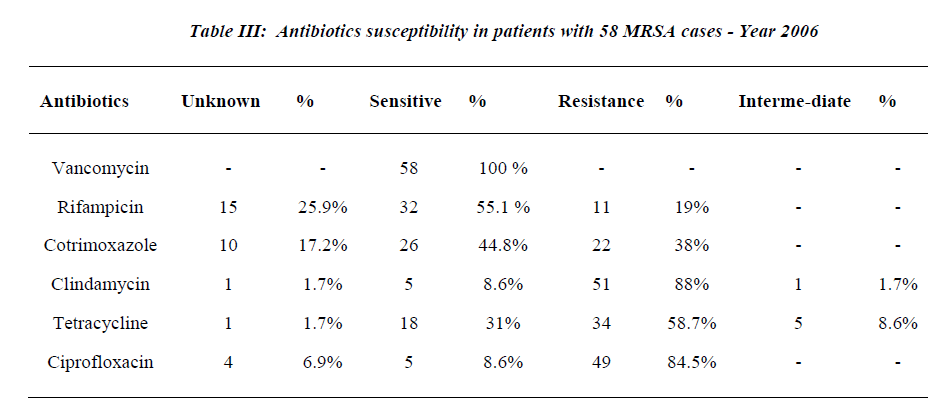
Twelve patients (20.7%) had 3 co-morbidities, 19 (32.8%) had 2 co-morbidities, 16 (27.5%) had 1 co-mor-bidity and 11 patients (19%) had no co-morbidities. Twenty-five patients (43.1%) were diabetics and 16 pa-tients (27.6%) had cardiac disease, 5 patients (8.6%) had renal disease. The total number of MRSA category of infections was 68 episodes. The majority occurred in the respiratory system where 30 infection episodes (44.1%) were observed and 10 patients (17.2%) were ventilated, followed by surgical site infection, skin subcutaneous tissue and bacteremia: 16 (23.5%), 12 (17.7%) and 6 (8.8%) respectively. Three patients (4.4%) had catheter related MRSA and 1 patient (1.5%) had urinary tract in fection with MRSA. All the MRSA isolates were sensi-tive to vancomycin (100%); susceptibility to Rifampicin was 55.1% while 19.0% were resistant to Rifampicin. Resistance to Cotrimoxazole and Clindamycin were ob-served in 38% and 88% respectively (See Tables 1-3 & Figure 1).
Discussion
MRSA is a notorious organism causing Infections mainly in Health Care Institutions [1,3-24]. Outbreaks of such infection in hospitals are also accelerated with marked increase in morbidity and mortality specially outbreaks in ICU setting [12,16]. This increase is accompanied with a high economic cost. Efforts are made to control these outbreaks [16].
Many institutions have reported an increase in the inci-dence of MRSA in recent years [3]; meanwhile other inst-itution reported a variation in the incidence. Zaman and Dibb reported during a 3-year period study the prevalence of MRSA showed only minor variation between 6.5% and 8.9% at King Khalid National Guard Hospital in Jeddah [17].
In our institution, in Riyadh, the trend was toward a de-crease in the incidence of MRSA. This is most likely due to the activation of the Infection Control Department im-plementing a strict surveillance continuous education for the Health Care Workers (HCW). stressing on the impor-tant role of handwashing and use of alcohol gel in almost every area in the hospital [7].
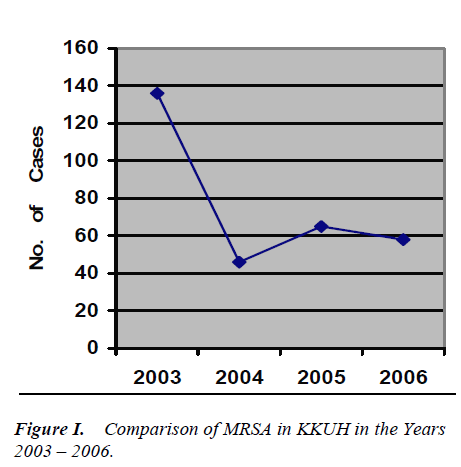
Finger printing feedback and active interaction with hos-pital staff specifically with the treating physician had likely contributed to the decreasing trend noticed at KKUH. The number of MRSA isolate was 461 in 2003 which subsequently dropped to 136 in 2004, 65 in 2005 and was 58 in 2006 during the study year.
The majority of our patients were above 60 years(46.6%). This is in agreement with Madani [20] who reported 36% at King Fahad Hospital (KFH) in Jeddah [20]. In this study, the age group between 31-60 was only (27.6%). Meanwhile, Madani and his co- workers [20] have re-ported higher number (33 and 46%) at King Abdulaziz University Hospital (KAUH) in Jeddah and KFH in Jed-dah respectively.
The gender description was 74.1% male and 25.9% fe-male. A similar sex description was reported from KFH in Jeddah. Bukhari and Abdelhadi [24] found male 63% and female 37%. Zamann R. reported 69% were male [17] Madani et al have reported 73% male and 27% fe-male [20]. Twenty-eight per cent (28%) of our MRSA isolates were cultured from the skin and subcutaneous tissues. This is in disagreement with other studies, Buk-hari et al [23] and Madani et al [20].
The epidemiology of MRSA has continued to evolve since its first appearance in the 1960s. At that time, MRSA were found sporadically and most of MRSA iso-lates were susceptible to some orally administered antibi-otic like clindamycin, macrolides and tetracycline [27]. In this study, 55% of MRSA isolates were susceptible to rifampicin, 38% resistant to cotrimoxazole, 59% were resistant to tetracycline and only 9% were susceptible to clindamycin.
Vancomycin has been the drug of choice for MRSA in-fections for the past 2 decades [17]. Recent reports, how-ever, have described strains of MRSA that were interme-diately resistant to glycopeptide’s (glycopeptide’s-intermediate S. aureus (GISA) and that were associated with therapeutic failure of vancomycin. Such strains have been reported from Japan, the United States, Europe (France, the United Kingdom and Spain), and the Far East (Hongkong and Korea). The isolates from the United States, France, and strain Mu50 from Japan appear to have developed from preexisting MRSA infections. The isolation of such strains from several parts of the world suggests that GISA will continue to emerge worldwide, portending the emergence of MRSA strains for which there will be no effective therapy, a situation similar to that in the pre-antibiotic [19-21] era. Luckily in this study, none of the isolates proved to be a vancomycin resistant strain.
Risk factors that have been associated with MRSA acqui-sition include older age, prolonged hospitalization, prior antibiotic therapy [15,16-22], more severe underlying disease and degree of disability, surgical procedures, pres-ence in an ICU or burn unit, having a surgical – wound infection, intravascular devices, mechanical ventilation [23,24], tracheostomy, pressure ulcers, or exposure to other infected or colonized individuals. Not only does antibiotic therapy predispose to colonization to MRSA, but it also increases the risk of infection and invasive dis-ease, as demonstrated by this study where significantly more patients with MRSA infection than those with MRSA colonization received antibiotics prior to positive MRSA culture (95.7 % vs 70%; P<001). Other host fac-tors associated with progression from colonization to in-fection include recent prior hospitalization, preceding surgery or wound debridement, and the number of inva-sive procedures [28,29].
Approximately three quarters (77.6%) of cases repre-sented with infection and the remainder (22.4%) showed colonization. This high infection–to-colonization ratio has been observed by other authors, for instance, Madani and co-workers reported an infection rate of 73% [20].
In agreement with other authors [30,31], the mortality of patient with MRSA infection was high in this study, par-ticularly in patients with multiple co-morbidity factors. It could be demonstrated that patient with 2 co-morbidities have more chances to acquire MRSA of 53.5% compared to 27.5% of those with only one co-morbidity. Earlier publication pointed to less or at least comparable outcome of patient with MSSA and with MRSA Bacteremia [28,29]. In the last years, however, reports are more and more suggesting that MRSA Bacteremia is associated with significant higher mortality rate than MSSA Bac-teremia [32]. In our study, MRSA Bacteremia was found only in six patients and four of them who had other co-morbid condition died. Therefore, it is difficult to postu-late a direct relation to a single factor causing this high mortality.
References
- Abbasi K. Facing the microbial threat. BMU 1998; 317: 620.
- Kolif M, Fraser V. Antibiotic resistance in the intensive care unit. Ann Intern Med 2001; 134: 298-314.
- McDonald P, Mitchel E, Johnson H, Rossney A et al. Epidemiology of MRSA: The north/south study of MRSA in Ireland 1999. J Hosp Infect 2003; 54: 130.134.
- Duckworth G, Cookson B. Humphreys H, Heathcock R. Revised guidelines for the control of methicillin- resistant Staphylococcus aureus infection in hospitals. J Hosp Infect 1998; 39: 253-290.
- McCullock J. Risk management in infection control. Nursing Standard 1999; 13: 44-46.
- Baird VL, Hawly R. Methicillin-resistant Staphylococ- cus aureus (MRSA): there a need to change clinical practice? Inten Crit Care Nurs 2000; 16: 357-366.
- Nettleman MD. Trilla A, Fredrickson M, Pfaller M. As- signing responsibility: Using feedback to achieve sustained control of methicillin-resistant Staphylococ- cus aureus. Am J Med 1991; 91: 228S-232S.
- Jevons MP. “Celbenin” - resistant Staphylococci. BMJ 1961; 1: 124-125.
- Wolfgang Witte. International dissemination of antibi- otic resistant strains of bacterial pathogens. Infect Ge- netics Evol 2004; 4: 187-191.
- Kucers A, Crowe S, Grayson ML, Hoy J. Methicillin In: The use of antibiotics, 5th edn. Butterworth- Heinemann, Oxford, 1997; 19: 77-84.
- Soule BM, HHuskins WS. A global perspective on the past, present and future of nosocomial infection pre- vention and control. Am J Infect Cont 1997; 25: 289- 293.
- Wenzel RP, Reagan DR, Bertino JS et al. Methicillin- resistant Staphylococcus aureus outbreak: a concensus panel’s definition and management guidelines. Am J Infect Cont 1998; 26: 102-110.
- MacConnachie AM. New antibiotics for gram positive infections: Linezolid and combination quinapristin/ dalfopristin. Intensive Crit Care Nurs 1999; 15: 239-241.
- Suller MTE, Russell AD. Antibiotic and biocide resis- tance in methicillin- resistant Staphylococcus aureus and vancomycin-resistant enterococcus, J Host Infect 1999; 43: 281-291.
- Al-Masaudi SB, Russel AD, Day MU. Comparative sensitivity to antibiotics and biocides of methicillin- resistant Staphylococcus aureus strains isolated from Saudi Arabia and Great Britain. J Appl Bacteriol 1991; 7: 331-228.
- Haddad Q, Sobayo EI, Basit OB, Rotimi VO. Outbreak of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit.J Hosp. Infect 1993; 23: 211-222.
- Zaman R, Dibb WL. Methicillin resistant Staphylococ- cus aureus (MRSA) isolated in Saudi Arabia: Epidemi- ology and antimicrobial resistance patterns. J Hosp In- fect 1994; 26: 297-300.
- Appendix A-1. CDC Definition of Nosocomial infec- tions, From: Horan TC, Gaynes Rp. Surveillance of nosocomial infections. In: Hospital Epidemiology and Infection Control, 3rd ed., Mayhall CG, editor. Philadel- phia: Lippincott Williams and Williams, 2004: 1659- 1702.
- Alghaithy AA, Bilal NE, Gedebou M, Weily AH. Na- sal carriage and antibiotic resistance of Staphylococcus aureus isolates from hospital and non-hospital person- nel in Abha, Saudi Arabia. Trans R Soc Trop Med Hyg 2000; 94: 504-507.
- Madani TA, Al-Abdullah NA, Al-Sanousi AA, Ghab- rah TM, Afandi SZ, Bajunid HA. Methicillin-resistant Staphylococcus aureus in two tertiary care centers in Jeddah, Saudi Arabia. Infect Control Hosp Epidemil 2001; 22: 211-216.
- Van Belkum A, Vandenbergh M, Kessie G, Qadri SM, Lee G, van den Braak N, Verbrugh H, Al Ahdal MN. Genetic homogeneity among methicillin-resistant Stap- hylococcus aureus strains from Saudi Arabia. Microb Drug Resist 1997; 3: 36536-36539.
- Embil J, Almuneef M, Nicoll D, Makki S, Cunningham G, Wylie J, Nicolle L, Memish Z. Methicillin-resistant Staphylococcus aureus profiles oceans apart-Canadian and Saudi Arabian experiences. J Chemother 2001; 13: S28-S33.
- Bukharie HA, Abdelhadi MS, Saeed IA, Rubaish AM, Larbi EB. Emergence of methicillin-resistant Staphy- lococcus aureus as a community pathogen. Diagn Microbiol Infect Dis 2001; 40: 1-4.
- Bukharie HA, Abdelhadi MS. The epidemiology of methicillin-resistant Staphylococcus aureus at a Saudi University Hospital. Microb Drug Resist 2001; 7: 413-416.
- Austin TW, Austin MA, McAlear DE, Coleman BT, Osoba AO, Thqafi AO, Lamfon MA. MRSA prevalence in a teaching hospital in Western Saudi Arabia. Saudi Med J 2003; 24: 1313-1316.
- Tayfour M, Eris FN, Alanazi A. Comparison of antibi- otic susceptibility tests, plasmid profiles and restriction enzyme analysis plasmid DNA of methicillin suscepti- ble and resistant Staphylococcus aureus strains isolated from intensive care units. Saudi Med J 2004: 25.
- Brumsfill W, Hamilton Miller. MRSA N Engl Med 1989; 320: 1188-1196
- Hanifa YA, Hiramatu K Yokotat. Characterization of methicillin-resistant Staphylococcus aureus with noso- comial infections in the University Hospital, Kuala Lumpur J Hosp. Infect. 1992, 21, 15-28.
- Boyce JM. Patterns of methicillin-resistant Staphylo- coccus aureus prevalence. Infect Control Hosp Epide- miol 1991; 12: 79-82
- Foagali JL, Thong ML, Grant D. Ten year’s experience with methicillin- resistant Staphylococcus aureus in a large Australian hospital. Hosp Infect 1992; 20: 113-119.
- Romero-Vivas J, Rubio M, Fernandez C, Picazo JJ. Mortality associated with nosocomial bacteremia due to methicillin-resistant Staphylococcus aureus. Clin. Infect Dis 1995; 21: 1417-1423.
- Conterno LO, Wey SSb, Castelo A. Risk factors for mortality in Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol 1998; 19: 32-37.