ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2013) Volume 24, Issue 4
Prospective study on Clotting Factors in selected non-hematological malignancies.
1Department of Pathology, King George’s Medical University, Lucknow, Uttar Pradesh, India.
2Department of Radiation Oncology, King George’s Medical University, Lucknow, Uttar Pradesh, India.
3Department of Radiation Oncology, King George’s Medical University, Lucknow, Uttar Pradesh, India.
4Department of Pulmonary Medicine, King George’s Medical University, Lucknow, Uttar Pradesh, India.
- *Corresponding Author:
- Ashutosh Kumar
Post Graduate Department of Pathology
King George’s Medical University
Lucknow 226003, Uttar Pradesh
India
Accepted Date: July 27 2013
Citation: Yusuf M, Kumar A, Kushwaha R, Bhatt MLB, Abhijit Chandra A, Kant S, Siddiqui Z. Prospective study on Clotting Factors in selected non-hematological malignancies. Biomedical Research 2013; 24 (4): 445-448.
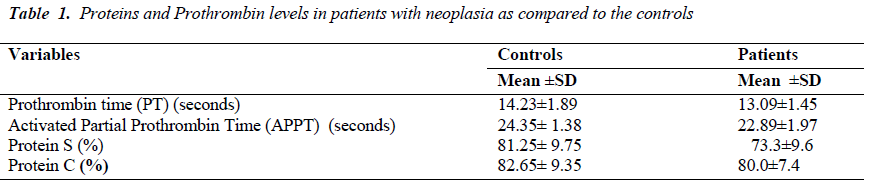
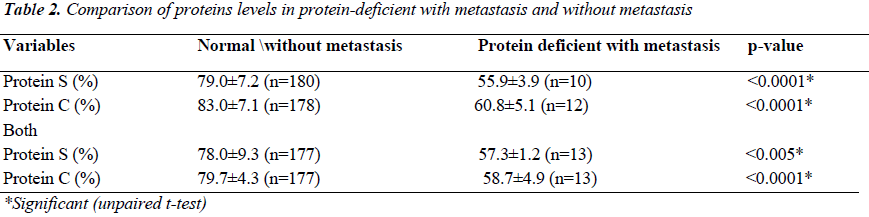
A hypercoagulable or prothrombotic state of malignancy occurs due to the ability of tumor cells to activate the coagulation system by producing and secreting procoagulant substances and inflammatory cytokines, and also by the physical interaction between tumor cell and blood cells (monocytes, platelets, and neutrophils) or vascular cells. A total of 190 Patients with carcinoma with and without metastasis from Surgical Gastroenterology, Radiation oncology Department, and from Pulmonary Medicine KGMU UP India, were studied in order to evaluate the presence and extent of hemostatic abnormalities in case of gastrointestinal carcinoma, lung carcinoma. The average prothrombin time in patients of gastrointestinal carcinoma, Lung carcinoma was less compared with control. Similarly, the average activated partial prothrombin time was also found to be less as compared to controls. The mean level of Protein S ranged from 45% to 98%. The mean level of Protein C ranged from 53% to 95%. The Protein C level was significantly lower (p<0.0001) in Protein C deficient patients with metastasis subjects without metastasis. Similarly, the Protein S level was significantly lower (p<0.0001) in protein S deficient patients with metastasis as compared to subjects without metastasis. The Protein C and protein S levels were also lower in those who were deficient with metastasis. Our study infers that epithelial malignancies have a subclinical prothrombotic state, progressing to thrombotic events that contribute significantly to morbidity and mortality in metastatic patients and we conclude that apart from hyperactive coagulation mechanism (as evidenced by relative shortening of PT and APTT). Protein C and Protein S deficiency also have a potential contributory role in promoting metastasis and thereby contributing to morbidity of cancer and its role in predicting thrombosis. . However, further studies are needed to strengthen the proposed mechanism.
Keywords
Protein C, Protein S, Prothrombotic state, Cancer, Metastasis, India
Introduction
Hypercoagulable or prothrombotic state of malignancy occurs due to the ability of tumor cells to activate the coagulation system by producing and secreting procoagulant substances and inflammatory cytokines, and also by the physical interaction between tumor cell and blood cells (monocytes, platelets, neutrophils) or vascular cells [1]. Other mechanisms of thrombus promotion in malignancy include nonspecific factors such as the generation of acute phase reactants and necrosis (e.g. inflammation), abnormal protein metabolism (e.g. paraproteinemia), and hemodynamic compromise (e.g. stasis). In addition, anticancer treatment (e.g. surgery/chemotherapy/hormone therapy) may significantly increase the risk of thromboembolic events by similar mechanisms, e.g., procoagulant release, endothelial damage, or stimulation of tissue factor production by host cells [2]. However, not all of the mechanisms for the production of a hypercoagulable state of cancer are entirely understood. In this study, we attempt to describe in brief the currently accepted concept about the pathophysiology of the hypercoagulable state of cancer, changes in antithrombotic mechanisms in malignancy and then assess the levels of certain natural anticoagulant proteins in patients with metastatic and non metastatic epithelial malignancy. The association between malignancy and thromboembolic disease is well established and has been recognized in medical literature for at least 135 years. The relationship between tumor growth and the activation of blood coagulation was first established in 1865 when Professor Armand Trousseau first described the clinical association between primary or idiopathic venous thromboembolism and occult malignancy [3]. But it is only in the last two decades that significant advancement in this field has been achieved. Trousseau described 182 cases of primary thrombophlebitits occurring in occult malignancies with pathological findings such as formation of venous and arterial platelet rich micro thrombi in the vasculature. Since the original Trousseau paper, numerous clinical and pathological observations confirmed the increased risk of thrombosis in patients with cancer and highlighted the involvement of activated coagulation and fibrinolytic system in genesis of cancer [4-9].
Material and Methods
A total of 190 patients with carcinoma with and without metastasis from Surgical Gastroenterology, Radiation Oncology Department, and from Pulmonary Medicine of KGMU, UP, India were studied in order to evaluate the presence and extent of hemostatic abnormalities in case of gastrointestinal carcinoma and lung carcinoma. Patients with cardiovascular diseases, diabetes, HIV or any infectious disease, previous malignancy or previous thromboembolic events were excluded from the study. In all the patients, hemostatic markers associated with extrinsic, intrinsic pathway as well as Protein C, Protein S estimation were evaluated prior to surgical intervention. 3 ml citrated blood was collected from each patient as per standard protocol. Platelet-poor plasma (PPP) was obtained by 15 - 20 minute of centrifugation at 3000 g.
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) were noted according to standard methods as described by Lewis SM [14] . Plasma was transferred to eppendrofs tube without delay, frozen and stored at -800C for future use. The study was approved by institutional ethic committee of K.G. Medical University, UP Lucknow, India. Written informed consent was obtained from each patient. Protein C and Protein S estimation were done by semiautomatic Hemostar Coagulometer.
Statistical analysis
The results are expressed as mean ± standard deviation (SD) and percentages with 95% confidence interval (CI) and range. For continuous data, the independent-sample t test was used to compare two means. A P<0.05 was considered statistically significant.
Results
The average prothrombin time in patients of gastrointestinal carcinoma and lung carcinoma was found to be less as compared to controls. Similarly, the average activated partial prothrombin time was also less as compared with the controls [Table-1]. The mean level of Protein S ranged from 45% to 98%. The mean level of Protein C ranged from 53% to 95%. Out of the total 190 patients, 12 were Protein C deficient and 10 were Protein S deficient. However, 13 patients were deficient of both the Protein C and Protein S. The level of Protein C was significantly lower (p<0.0001) in Protein C deficient patients with metastasis compared to without metastasis. Similarly, the Protein S level was found to be significantly lower (p<0.0001) in protein S deficient patients with metastasis as compared to patients without metastasis. The Protein C and S levels were also lower in those who were deficient with metastasis [Table 2].
Discussion
Cancer cells can activate coagulation directly through an interaction with platelets and/or clotting and fibrinolytic systems to generate thrombin. Clotting activation may be considered as a special type of inflammatory reaction to stimuli such as vessel wall damage, or intravascular cell aggregation or entry in blood of abnormal cells such as tumor cells. The level of coagulation factors and natural anticoagulant proteins synthesized in liver are valuable in assessing pathogenetic mechanism of thrombosis in malignancy. Protein C in the specimen is activated by addition of snake venom contained in Protein C activator reagent. Activated Protein C inhibits Factor V and Factor VIII contained in the addition Protein C deficient plasma. This inhibition reaction result in prolongation of the subsequent APTT test. The degree of prolongation of clotting time corresponds to the Protein C concentration in the specimen. Plasma specimen is diluted and mixed with specific snake venom (Protein S activator reagent) and Protein S deficient plasma. Activated protein C proteolytically cleaves factor VA and factor VIIIA generated during the activation of coagulation cascade in the subsequent APTT test. Protein S acts as a cofactor in accelerating this reaction. The degree of prolongation of clotting time corresponds to the Protein S concentration in the specimen. Many studies in past have tried to establish the role of Protein C and Protein S in different cancers, but only few of them have tried to compare their significance in metastatic and non-metastatic settings. In this study we tried to assess the role of anticoagulant markers (mainly restricting to levels of Protein C and Protein S) with intent to look for any prognostic or predictive significance of the same in patients with and without metastasis. The mean level of Protein S ranged from 45% to 98%. The mean level of Protein C ranged from 53% to 95%. Out of the total of 190 patients, 12 were Protein C deficient and 10 were Protein S deficient. However, 13 patients were deficient of both Protein C and S. The Protein C level was significantly lower (p<0.0001) in Protein C deficient patients with metastasis compared to patients without metastasis. Similarly, the Protein S level was significantly lower (p<0.0001) in protein S deficient patients with metastasis as compared to patients without metastasis. The Protein C and S levels were also lower in those who were deficient with metastasis. Contrary to our results, In 1999, Gouin-Thibault et al. [10] and Yigit et al. in 2008 [11] reported that Protein C and S levels decreased in breast cancer cases. Protein S levels were reportedly lower than in controls as reported by Yigit et al. [11]. This was further complimented in 1988 [12] and it was shown that Protein C and S levels were significantly decreased in breast cancer and concluded that the breast cancer patients are under the risk of thromboembolism even without taking any form of treatment like surgery, chemotherapy or hormone therapy. Similar results were reported in studies on colorectal patients. In 1992, Ellis et al. [13] reported decreased Protein C activity in colon cancer and this decrease had been found to be interrelated with deep vein thrombosis. Consistent with the shift of the Hemostasis balance toward hypercoagulation in cancer, several studies have shown reduced levels of natural inhibitors of coagulation such as antithrombin, Protein C and Protein S in plasma of cancer patients. These decreased levels of inhibitors might result from an increased consumption as a consequence of the activation of coagulation or from a defective hepatic synthesis or both. In this study we discuss and analyze the mechanism of thrombosis in malignancy, change in hemostatic parameters and their relation to cancer progression.
Conflict of interest
None declared
Author’s Contribution
Conception, design, analysis and interpretation of data by Prof Ashutosh Kumar, Drafting the article or revising it critically for important intellectual content by Mohd. Yusuf, Zainab Siddiqui and final approval of the version to be published by Prof. Ashutosh Kumar, Madan Lal Brahma Bhatt, Abhijit Chandra and Surya Kant.
Acknowledgement
We acknowledge UPCST for providing funds to conduct this study and we are thankful to all laboratory staff of Coagulation and Hemostasis lab of KG Medical University Lucknow.
References
- Hillen HF. Thrombosis in cancer patients. Ann Oncol 2000; 11 (Suppl 3): 273-276.
- Wojtukiewicz MZ, Tang DG, Ciarelli JJ, Nelson KK, Walz DA, Diglio CA, et al. Thrombin increases the metastatic potential of tumor cells. Int J Cancer 1993; 54: 793-806.
- Wojtukiewicz MZ, Sierko E, Zacharski LR, Zimnoch L, Kudryk B, Kisiel W. Tissue factor-dependent coagulation activation and impaired fibrinolysis in situ in gastric cancer. SeminThrombHemost 2003; 29: 291-300.
- Gunji Y, Lewis J, Gorelik E. Fibrin formation inhibits the in vitro cytotoxic activity of human natural and lymphokine activated killer cells. Blood Coagul Fibrinolysis 1990; 1: 663-672.
- Bruhn HD, Zurborn KH. Influences of clotting factors (thrombin, factor XIII) and of fibronectin on the growth of tumor cells and leukemic cells in vitro. Blut 1983; 46: 85-88.
- Wojtukiewicz MZ, Sierko E, Rak J. Contribution of hemostatic system to angiogenesis in cancer. Semin Thromb Hemost 2004; 30: 5-20.
- Trousseau A. Phlegmasiaalbadolens. In: Clinique Medicale de l’HotelDieu de Paris, 2nd , Vol. 3. Paris: Ballière; 1865; p. 654-712.
- Sengül N, Demirer S, Yerdel MA, Terzioğlu G, Akin B, Gürler A, et al. Comparison of coagulation parameters for healthy subjects and Behçet disease patients with and without vascular involvement. World J Surg. 2000; 24 : 1584-1588.
- Ozyilkan O, Baltali E, Ozdemir O, Tekuzman G, Kirazli S, Firat D. Haemostatic changes; plasma levels of alpha 2 antiplasmin-plasmin complex and thrombin antithrombin III complex in female breast cancer. Tumori 1998; 84: 364-367.
- Gouin-Thibault I, Samama MM. Laboratory diagnosis of the thrombophilic state in cancer patients. Semin ThrombHemost 1999; 25: 167-172.
- Yigit E, Gönüllü G, Yücel I, Turgut M, Erdem D, Cakar B. Relation between hemostatic parameters and prognostic/predictive factors in breast cancer. Eur J Intern Med 2008; 19(8): 602-607.
- Ellis CN, Boggs HW, Slagle GW, Cole PA, Coyle DJ, Blakemore WS. Protein C activity, stage of disease, and vascular thrombosis in colon carcinoma. Am J Surg 1992; 163: 78-81.
- Ozyilkan O, Baltali E, Ozdemir O, et al. Haemostatic changes; plasma levels of alpha 2-antiplasmin-plasmin complex and thrombinantithrombin III complex in female breast cancer. Tumori. 1998; 84(3): 364-367.
- A practical Book of Hematology by SM Lewis and Deice third edition; 2006