ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 2
Protective effects of dexpanthenol against acetaminophen-induced hepatorenal damage
1Department of Internal Medicine, Adnan Menderes University School of Medicine, Aytepemevkii Merkez/Aydin, Turkey
2Department of Emergency, Adnan Menderes University School of Medicine, Aytepemevkii Merkez/Aydin, Turkey
3Department of Biochemistry, Adnan Menderes University School of Medicine, Aytepemevkii Merkez/Aydin, Turkey
4Department of Histology, Adnan Menderes University School of Medicine, Aytepemevkii Merkez/Aydin, Turkey
5Department of Biostatistics, Adnan Menderes University School of Medicine, Aytepemevkii Merkez/Aydin, Turkey
6Department of Pharmacology, Adnan Menderes University School of Medicine, Aytepemevkii Merkez/ Aydin, Turkey
- *Corresponding Author:
- Hilal Bektas Uysal
Adnan Menderes University
School of Medicine
Department of Internal Medicine
Aytepemevkii Merkez/Aydin, Turkey
Accepted on June 20, 2016
Acetaminophen (paracetamol, APAP) has a worldwide usage. Nevertheless, overdosing may induce severe toxicity. Dexpanthenol (DXP) is the alcohol provitamin form of vitamin B5 with antiinflammatory and antioxidant efficacy. In the present study, the biochemical and histological protective effects of DXP against acetaminophen-induced oxidative hepatorenal damage were examined. Rats were divided into the following groups: healthy control (HG); APAP (AG (APAP Group), 1500 mg/kg, orally); DXP (DXG, 500 mg/kg, intraperitoneally); APAP+N-acetylcysteine (NAC) (ANG, 100 mg/kg, intraperitoneally); APAP+DXP (ADXG) and APAP+NAC+DXP (ANDXG) groups. Liver and kidney function tests, oxidant/antioxidant parameters and histological assessment were performed. In the AG group, marked hepatorenal damage occurred with the significant elevation of kidney (urea and creatinine) and liver (alanine aminotransaminase, aspartate aminotransaminase lactate dehydrogenase) function tests, and oxidative stress markers such as malondialdehyde, myeloperoxidase and nitric oxide when compared with the HG group (p<0.05). Concurrently, an apparent decrease in catalase and glutathione levels was determined in the AG group (p<0.05). In the ADXG group, DXP significantly decreased oxidant levels in both liver and kidney tissues while increasing the antioxidant levels when compared with the AG group (p<0.05). The resultant histological changes were improved and almost normal organ structures in the ADXG group. Furthermore, the biochemical and histological assessment results revealed that DXP has nearly the same hepatoprotective efficacy with NAC. In renal tissues, when all groups were compared against the AG group, there was a statistically significant difference between all groups (p<0.001) except the ANG group. The histological sections of the ANG group were nearly the same, with the AG group indicating the inadequate nephroprotective effect of NAC. In the light of these findings, we think that DXP may be used in daily clinical practice of acute hepatorenal APAP-induced toxicity. However, further studies are needed to illuminate its efficacy.
Keywords
Acetaminophen, Dexpanthenol, Hepatotoxicity, Nephrotoxicity, Rat.
Introduction
Acetaminophen (paracetamol, APAP) has worldwide usage for its analgesic and antipyretic efficacy. APAP has a few side effects but is generally safe when used at therapeutic levels. Nevertheless, an over dosage for both therapeutic purposes and for suicide may induce severe toxicity [1]. In both humans and animals, APAP can cause hepatic necrosis and renal failure [2-4]. Nearly 114,775 cases of APAP intoxication have been reported in the United States in 2014 [5]. Nearly 50% of all acute liver failure cases are on account of APAP toxicity, with results of 30% mortality [6]. APAP-induced hepatotoxicity has been investigated in many studies, and it is a well-defined topic with various aspects. Renal damage is rarely observed when compared with hepatotoxicity [7]. In nearly 1-2% of APAP-overdosed patients, renal insufficiency occurs [8].
APAP-induced organ damages have similar mechanisms, and only minor differences may appear between hepatic and renal manifestations. Toxicity occurs because of the toxic and reactive metabolite of APAP. When taken at prescribed doses, APAP is metabolized in the liver by glucuronidation and sulphation, and is excreted via urine. Under normal circumstances, endogenous glutathione (GSH) detoxifies the reactive metabolite N-acetyl-p-benzo-quinoneimine (NAPQI). Nevertheless, in APAP toxicity, sufficient NAPQI detoxification cannot be processed because the stocks of GSH are depleted. Moreover, NAPQI covalently binds to intracellular proteins, leading to renal and hepatic injury [9,10]. The progenitor mechanism of toxicity responsible for causing oxidative stress, lipid peroxidation and depletion of protein thiols, is thought to be the covalent binding [11,12]. On account of the key roles of increasing oxidative stress with depleted stores of GSH, and lipid peroxidation in the development of life-threatening APAP-induced toxicity, several individual antioxidant vitamins have been studied with the aim to prevent these damages [13-15]. In APAP toxicity, acting as an antioxidant system, hepatic GSH content is very important for protection against cellular injury. N-acetylcysteine (NAC) potentiates hepatic synthesis of GSH and supplies GSH stores. However, NAC is known to decrease hepatotoxicity, and its usefulness in the treatment of nephrotoxicity is unclear [3,16]. Additionally, NAC has some limits in utilization, such as the importance of application time after APAP toxicity, and the narrow therapeutical window [17]. Therefore, new treatment modalities for APAP-induced toxicity are still the subject of interest.
Dexpanthenol (DXP, Provitamin B5) is the alcohol provitamin form of vitamin B5. It is converted to Panthotenic acid (PA) in rat and mammalian tissues [18,19]. DXP has anti-inflammatory and antioxidant efficacy by increasing the levels of reduced GSH, coenzyme A (CoA), and stimulating synthesis of adenosine-5’-triphosphate (ATP) in cells [20,21]. It has been shown in several studies that PA and its derivatives have protective effect against the damage induced by reactive oxygen species in the tissues [22,23]. Within this entire efficacy, DXP has a key role in cellular repair capability and cellular defense mechanisms against lipid peroxidation and oxidative stress [24]. As lipid peroxidation and oxidative stress were reported to play major roles in the pathogenesis of APAP-induced damage, DXP may be a logical approach in the prevention and treatment of APAP-induced organ damage. According to our knowledge, this is a novel study referring both the histological and biochemical effects of DXP in APAP-induced hepatorenal toxicity. The aim of our study was to examine whether DXP has a preventive effect on oxidative hepatorenal organ injury induced with a high dose of APAP, and also compared with NAC therapy.
Materials and Methods
Animals
A total of 48 female albino Wistar rats weighing 220–240 g were obtained from Adnan Menderes University Experimental Animal Unite, Aydin, Turkey. The animals were retained at constant room temperature (22°C ± 1°C) with a 12/12-hr light/ dark cycle in a colony room and allowed free access to food and water. All animal care and experimental procedures were in accordance with the NIH Guide for Care and Use of Laboratory Animals. The study was approved by the local animal ethics committee of Adnan Menderes University, Turkey (HADYEK, Number: 64583101/2014/020).
Chemical substances
DXP (Bepanthene®, Bayer, Istanbul, Turkey), APAP (Tamol®, Sandoz, Istanbul, Turkey) and NAC (Asist®, Husnu Arsan, Istanbul, Turkey) were obtained from the local pharmacy store to mimic the real clinical exposure and application situations.
Experimental procedure
Experimental animals were fasted overnight and divided into six different groups (eight in each group) randomly as: HG, APAP group (AG, 1500 mg/kg, orally), DXP group (DXG, 500 mg/kg, intraperitoneally), APAP+NAC group (ANG, 100 mg/kg, intraperitoneally), APAP+Dxp group (ADXG) and APAP+NAC+Dxp (ANDXG) group. Only the DXP-administered group was formed to be sure whether DXP by itself caused any harmful effect on study parameters. Saline was administered by intraperitoneal route to the HG and AG groups. One and a half hours before the administration of drugs and saline, APAP was administered orally to all groups excluding HG and DXG. Although it has been reported that the rats are more resistant to develop APAP-induced liver injury model than mice [25], the APAP dose and time period for collection of blood and tissue sampling after drug administration was determined after testing the rat strains and interaction with pharmaceutical formulation of paracetamol in our preliminary studies. Additionally, we also took the study of Elshazly et al. [26] into account about dosing, sampling and establishment of liver injury. There was no animal loss in the course of our study. After 12 hr of APAP administration, the animals were killed under anesthesia of xylasine and ketamine (5 mg/kg and 50 mg/kg, respectively). Blood was taken by intracardiac puncture, and the excised livers and kidneys were fixed in 10% neutral-buffered formalin solution.
Blood analyses
Blood samples were centrifuged at 3000 rpm for 7 min in a cold centrifuge. Serum was stored at −85°C for subsequent studies. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) liver enzymes as an indicator of cell necrosis, urea and creatinine as kidney function tests and lactate dehydrogenase (LDH) as a tissue injury marker, were determined with a chemiluminescent assay by a routine autoanalyzer (Architect C8000, Abbott, IL, USA).
Preparation of tissue homogenates
Specimens from the liver and kidney were weighed and homogenized separately with tissue homogenizer (PRO 250 Scientology Inc., Monroe, CT USA). For the estimation of tissue GSH, malondialdehyde (MDA), nitric oxide (NO; nitrite +nitrate) levels, the activities of catalase (CAT) and myeloperoxidase (MPO), tissues were homogenized in phosphate buffer saline (PBS) 50 mM pH 7.4. The crude tissue homogenate was centrifuged at 10000 rpm, for 15 min in an ice-cold centrifuge, and the supernatant was stored at −85°C.
Malondialdehyde
The production of MDA and consequently, lipid peroxidation, was determined in the tissues by using the standard method [27]. MDA forms a colored complex in the presence of thiobarbituric acid, which is detectable by measurement of absorbance at a wavelength of 532 nm. Absorbance was measured with a Shimadzu UV-160 spectrophotometer; 1,1′, 3, 3′ Tetra ethoxypropane was used as a standard, and the results were expressed as μmol/g wet tissue.
Myeloperoxydase
The activity of tissue-associated MPO was determined as reported by Suzuki et al. [28]. MPO activity was expressed in U/g wet tissue.
Glutathion
The content of GSH in tissue supernatants was measured according to the method of Beutler [29]. The absorbance was measured at 412 nm wavelength using a Shimadzu UV-160 spectrophotometer. The GSH concentration was determined using standard aqueous solutions of GSH. Results were expressed as μM/g wet tissue.
Catalase
The Aebi method was used to measure the CAT activity in the tissue [30]. The reduction rate of H202 was observed at 240 nm for 30 sec at room temperature. CAT activity was expressed in U/g wet tissue.
Nitric oxide
NO (nitrite+nitrate) was assayed by a modification of the cadmium-reduction method of Navarro-Gonzalves et al. [31]. The nitrite produced was determined by diazotization of sulfanilamide and coupling to naphthylethylenediamine. The samples were analyzed spectrophotometrically using a microplate reader, and quantified automatically against a KNO3 standard curves, and the results were expressed as μmol/g wet tissue.
Histological examination
After routine tissue processing, 5 micrometers-thick slices were obtained from the paraffin blocks of both liver and kidney tissues. The obtained sections were stained by hematoxylin and eosin for histological examination under light microscope (Olympus X20), and photographed with a digital camera.
Statistical analysis
All data were analyzed by using the PASW Statistics V18 software package. The Kolmogorov-Smirnov test was used to assess the normality of numeric variables. The results are defined as Mean ± Standard Deviation (SD). Differences between groups were obtained by using one-way analysis of variance (ANOVA) tests with Tukey-Kramer HSD as post- ANOVA tests. Significance was expressed at p ≤ 0.05.
Results
Results of hepatic and renal oxidant/antioxidant parameters
In Tables 1 and 2, the levels of oxidant/antioxidant parameters measured in both liver and kidney tissues are shown. In hepatic tissues, when HG, DXG, ANG, ADXG, ANDXG groups were compared against the AG group, in terms of oxidant/antioxidants, a statistically significant difference was identified between all groups (p<0.001).
| Groups | MDA µmol/g protein | MPO U/g protein | NO µmol/g protein | |||
|---|---|---|---|---|---|---|
| Hepatic | Renal | Hepatic | Renal | Hepatic | Renal | |
| HG | 0.21 ± 0.01* | 0.22 ± 0.01* | 46.57 ± 15.93* | 24.23 ± 3.87* | 2.75 ± 0.21* | 1.98 ± 0.08* |
| AG | 0.47 ± 0.05 | 0.48 ± 0.04 | 297.77 ± 76.87 | 105.85 ± 13.94 | 3.44 ± 0.32 | 3.16 ± 0.28 |
| DXG | 0.21 ± 0.01* | 0.21 ± 0.01* | 51.58 ± 18.03* | 23.27 ± 3.28* | 2.66 ± 0.25* | 1.97 ± 0.07* |
| ANG | 0.19 ± 0.01* | 0.47 ± 0.05 | 57.22 ± 26.40* | 100.80 ± 11.95 | 2.14 ± 0.11* | 3.11 ± 0.23* |
| ADXG | 0.19 ± 0.02* | 0.19 ± 0.01* | 55.23 ± 28.94* | 25.15 ± 4.17* | 2.13 ± 0.10* | 2.09 ± 0.09* |
| ANDXG | 0.18 ± 0.02* | 0.18 ± 0.02* | 56.40 ± 24.20* | 24.03 ± 5.32* | 2.12 ± 0.09* | 2.08 ± 0.09* |
Table 1. The comparison of oxidant levels in both rat hepatic and renal tissues.
| Groups | GSH µmol/g protein | CAT U/g protein | ||
|---|---|---|---|---|
| Hepatic | Renal | Hepatic | Renal | |
| HG | 14.925 ± 2.63* | 14.20 ± 1.97* | 35.91 ± 11.05ˠ | 35.15 ± 7.19* |
| AG | 7.662 ± 1.08 | 7.26 ± 1.16 | 9.93 ± 6.18 | 10.51 ± 3.47 |
| DXG | 14.175 ± 1.98* | 13.26 ± 1.52* | 35.91 ± 9.69ˠ | 33.60 ± 7.53* |
| ANG | 13.16 ± 1.37* | 8.02 ± 1.01 | 26.67 ± 3.28ˠ | 10.46 ± 3.93 |
| ADXG | 13.32 ± 1.07* | 13.78 ± 2.08* | 26.93 ± 3.02ˠ | 29.53 ± 3.55* |
| ANDXG | 13.46 ± 1.10* | 13.72 ± 1.98* | 26.95 ± 2.56ˠ | 29.92 ± 3.19* |
*p<0.01 was defined statistically significant. A comparison of the levels of MDA, MPO and NO of each group against the AG group, based on one-way ANOVA tests with Tamhane multiple comparisons.
ˠp<0.05 was defined statistically significant. A comparison of the levels of MDA, MPO and NO of each group against the AG group, based on one-way ANOVA tests with Tamhane multiple comparisons.
Table 2. The comparison of antioxidant levels in both rat liver and kidney tissues.
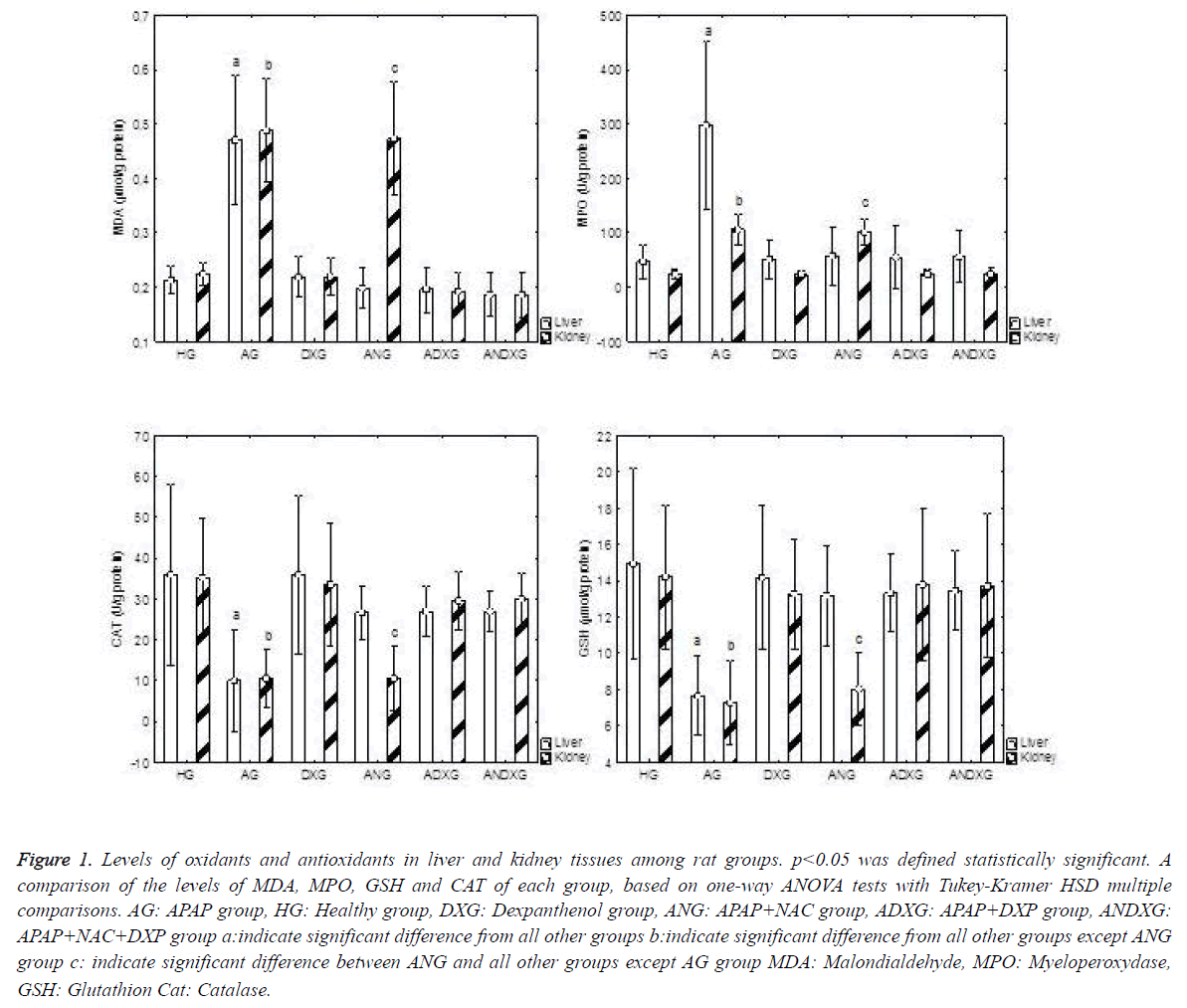
An apparent increase in oxidant parameters, and concurrently an apparent decrease in antioxidant levels were determined in the AG group. There was no statistically significant difference between the ANG and ADXG groups (p>0.05), between the ANG and ANDXG groups (p>0.05), and between the ADXG and ANDXG groups (p>0.05) in terms of oxidant/antioxidant levels. In renal tissues, when all groups were compared against the AG group, there was a statistically significant difference between all groups (p<0.001) except the ANG group. There were significant differences between the HG, DXG, ADXG and ANDXG groups in terms of oxidant and antioxidant levels when compared with the ANG group (p<0.001). There was no statistically significant difference (p>0.05) between the ADXG and ANDXG groups as shown in Figure 1.
Figure 1. Levels of oxidants and antioxidants in liver and kidney tissues among rat groups. p<0.05 was defined statistically significant. A comparison of the levels of MDA, MPO, GSH and CAT of each group, based on one-way ANOVA tests with Tukey-Kramer HSD multiple comparisons. AG: APAP group, HG: Healthy group, DXG: Dexpanthenol group, ANG: APAP+NAC group, ADXG: APAP+DXP group, ANDXG: APAP+NAC+DXP group a:indicate significant difference from all other groups b:indicate significant difference from all other groups except ANG group c: indicate significant difference between ANG and all other groups except AG group MDA: Malondialdehyde, MPO: Myeloperoxydase, GSH: Glutathion Cat: Catalase.
Results of hepatic and renal function tests
Table 3 shows the serum ALT, AST, LDH, urea and creatinine results of the rat group. Serum transaminases were significantly higher in the AG group when compared with the HG group (p<0.001), indicating hepatic damage. In the ANG, ADXG and ANDXG groups, after DXP or NAC administration, serum transaminase levels decreased significantly (p<0.001). When compared with the AG group, LDH levels were statistically significant in all groups (p<0.05). There was no significance (p>0.05) between the ANG, ADXG and ANDXG groups. As pointing to a nephrotoxicity, serum urea and creatinine levels were increased significantly in the AG group (p<0.001). After NAC administration, no significant difference was observed in the ANG group when compared with the AG group (p>0.05). There was no statistically significant difference between the ADXG and ANDXG groups (p>0.05), but, the HG, DXG, ADXG and ANDXG groups were statistically different when compared with the ANG group (p<0.001).
| Groups | ALT (U/L) | AST (U/L) | LDH (U/L) | UREA (mg/dl) | CREAT (mg/dl) |
|---|---|---|---|---|---|
| HG | 33.75 ± 3.77* | 132.87 ± 20.69* | 352.25 ± 112.37* | 33.62 ± 3.54* | 0.51 ± 0.04* |
| AG | 89.62 ± 28.42 | 253.75 ± 43.11 | 908.87 ± 397.60 | 108.75 ± 18.49 | 1.16 ± 0.20 |
| DXG | 33.12 ± 3.90* | 121.00 ± 18.02* | 358.00 ± 96.59* | 34.12 ± 4.94* | 0.54 ± 0.05* |
| ANG | 50.75 ± 7.57* | 169.25 ± 40.08* | 586.62 ± 134.64* | 99.25 ± 6.71 | 1.02 ± 0.12 |
| ADXG | 48.62 ± 6.16* | 148.37 ± 44.27* | 593.00 ± 143.51* | 41.625 ± 5.62* | 0.56 ± 0.06* |
| ANDXG | 45.00 ± 6.74* | 153.25 ± 23.26* | 530.75 ± 119.30* | 37.37 ± 3.96* | 0.52 ± 0.07* |
Table 3. liver and kidney function tests.
Results of histological examination
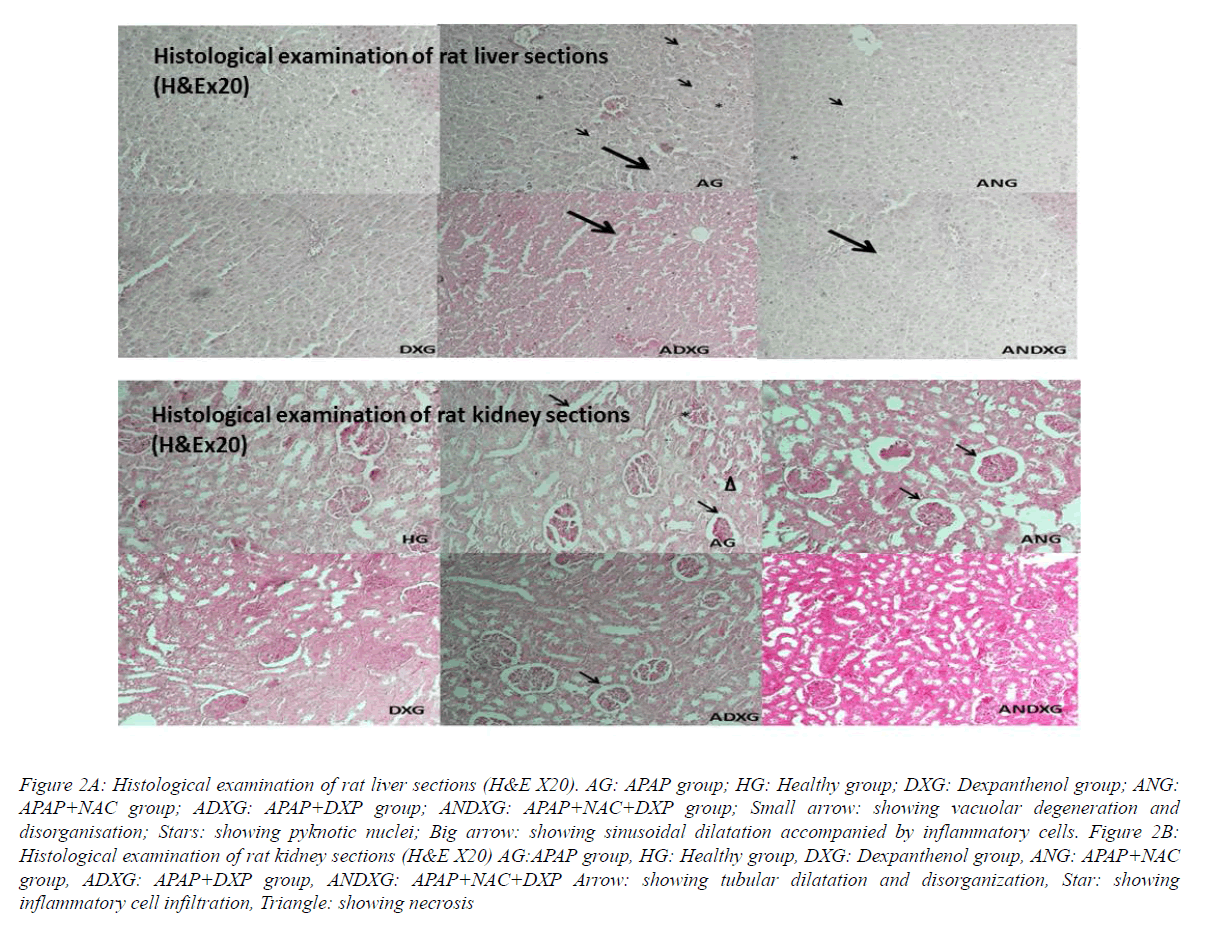
In the liver sections of the HG group’s rats, there was normal hepatic histology (Figure 2A). After APAP administration in the AG group, disorganization in hepatic lobules, sinusoidal dilatation concomitant with inflammatory cells, cellular degeneration with hepatic necrosis and pyknotic nuclei were monitored. In both NAC and DXP-treated rat groups, there were slight sinusal dilatations accompanied by partial cellular infiltrations. Except these tenuous changes, the hepatic sections appeared to look quite like near-normal hepatic histology in both, verifying the hepato protective effects of NAC and DXP, separately. In the assessment of DXP-alone administered liver sections, there was no histological change. The assessment of the liver sections of rats treated with NAC simultaneously with DXP was almost like near-healthy group liver structures, but not much different when compared with the DXP- and NAC-treated rat groups, respectively, pointing that DXP has no additional benefits with NAC. The tissue sections of the healthy group and the DXP-treated group of rats showed normal kidney histology with well-organized glomeruli and tubules (Figure 2B). In the AG group, tubular dilatation with damaged glomeruli accompanied with cellular infiltration and necrosis were observed. The NAC-treated ANG group sections were nearly the same as with the AG group except for cellular infiltration and necrosis, indicating the inadequate nephron-protective effect of NAC. In the ADXG group, there was nearly normal kidney structure with minimal focal tubular dilatations. The section of the ANDXG group was almost near-normal in kidney structure with no histological changes.
Figure 2A: Histological examination of rat liver sections (H&E X20). AG: APAP group; HG: Healthy group; DXG: Dexpanthenol group; ANG: APAP+NAC group; ADXG: APAP+DXP group; ANDXG: APAP+NAC+DXP group; Small arrow: showing vacuolar degeneration and disorganisation; Stars: showing pyknotic nuclei; Big arrow: showing sinusoidal dilatation accompanied by inflammatory cells. Figure 2B: Histological examination of rat kidney sections (H&E X20) AG:APAP group, HG: Healthy group, DXG: Dexpanthenol group, ANG: APAP+NAC group, ADXG: APAP+DXP group, ANDXG: APAP+NAC+DXP Arrow: showing tubular dilatation and disorganization, Star: showing inflammatory cell infiltration, Triangle: showing necrosis
Discussion
Our study aimed to examine the protective efficacy of DXP on hepatorenal oxidative damage induced by high doses of APAP in rat tissues. We also compared the efficacy of DXP with standard NAC therapy. Furthermore, whether NAC and DXP have an additive effect on APAP toxicity was investigated both biochemically and histopathologically.
In APAP toxicity, because of the rise in consumption by increasing APAP metabolites, the store of GSH gets depleted. These increasing APAP metabolites interfere with proteins by covalent binding; impair their functions and cause apoptosis and necrosis. This imbalance between oxidant/antioxidant systems leads to oxidative stress in tissues and finally organ failure begins [32,33]. Depletion of GSH stores is one of the progenitor factors of lipid peroxidation in APAP-induced toxicity. Therefore, reagents that are capable of increasing the content of GSH and have antioxidant efficacy are still the subject of interest among researchers. In human and animal tissues, DXP has anti-inflammatory and antioxidant efficacy by increasing the levels of reduced GSH, CoA and stimulating ATP synthesis in cells [20,21].
In our study, 1.5 g/kg APAP induced marked hepatorenal damage 12 hr after administration. This toxicity is evidenced by the significant elevation of serum ALT, AST, LDH, urea and creatinine levels which are specific organ function tests. Biochemical results were congruent with the histopathological changes of oxidative damage in the hepatic and renal tissues. Simultaneously, the comparison of the AG and HG groups revealed that APAP-induced toxicity also led to the elevation of oxidative stress indicators (MDA and MPO), and reduction in the levels of antioxidants (GSH and CAT) both in hepatic and renal tissues. These experimental results were consistent with similar studies on APAP toxicity [34-37].
Lipid peroxidation is the process of cell damage, and cell death is a common consequence of this system. The end product of lipid peroxidation is MDA, and it acts as an indicator of oxidative damage. By the cross-link formation and interaction with membrane lipids, MDA leads to severe damage [38]. Additionally, MPO is the other oxidant marker elevated in situations of oxidative damage. MPO is found in neutrophils and macrophages, and catalyzes the production of toxic hypochlorous acid. This toxic product is involved in hydroxyl radical formation [13,39]. The results of our study revealed a significant increase in both hepatic and renal tissue MDA and MPO levels in APAP-treated rats, indicating lipid peroxidation and, consequently, hepatorenal damage. In many studies, DXP was reported to prevent cell damage produced by lipid peroxidation irrespective of the source of oxidative stress [22-24,40]. Consistent with the literature, after administration of DXP, in the ADXG group, MDA and MPO levels were significantly decreased near to levels of the healthy group when compared with the AG group. In the NAC-treated ANG group, hepatic MDA and MPO levels were significantly decreased near to normal when compared with the AG group (p<0.001). Additionally, there was no statistically significant difference among the ANG, ADXG and ANDXG groups, indicating that there was no superior or additive efficacy of NAC with DXP in APAP-induced hepatotoxicity. However, strikingly, there was no significant difference between ANG and AG groups in terms of renal MDA and MPO levels (p>0.05). Consistent with other studies [3,41], in our study, NAC was not protective against renal oxidative stress induced by APAP.
It is known that the protective antioxidant efficacy of DXP against lipid peroxidation was due to the promotion of synthesis of CoA in cells. The increased content of CoA in the cell promotes the reparation of cell membranes by increasing the biosynthesis of phospholipids and cholesterol [22,24]. Furthermore, with an anti-inflammatory efficacy, DXP inhibits the release of MPO from polymorphonuclear cells [42]. As mentioned above, these protective efficacies were due to the metabolic products of PA which are the precursors of CoA. It has been shown that some derivatives of PA which are not CoA precursors cannot prevent oxidative damage [22,24]. Thus, we used DXP in our study as a CoA precursor derivative of PA.
Moreover, we have considered a marked elevation of nitric oxide (NO) in hepatic and renal tissues of rats in the AG group. In mitochondria, the product of NO and superoxide reaction is peroxynitrite. Peroxynitrite is a very toxic oxidant and also leads to tyrosine nitration. These reactive oxygen and nitrogen species directly cause mitochondrial DNA damage. The peroxynitrite formation and mitochondrial oxidative stress triggers the disruption of ATP synthesis [13,43]. Consistent with the data in the literature, NO levels were elevated in the AG group of our study indicative of the oxidative stress generated by APAP, and were lower individually in the hepatic sections of the ADXG, ANDXG and ANG groups due to the antioxidant efficacy of DXP and NAC. But in the renal sections, only in the ADXG and ANDXG groups were the NO levels decreased when compared with the AG group. In the ANG group, there was a decrease in renal levels of NO, but this cannot meet the statistically significant difference (p>0.05).
In several studies, it has been shown that while increasing the CoA content of the cells, DXP also increases the stores of GSH and ATP synthesis of the living cells [20,21,23]. As is very well known, GSH and GSH-dependent peroxidases are the major defence mechanisms against oxidative stress and lipid peroxidation [44]. While participating in the removal of lipid peroxidation products, GSH is also involved in a wide range of metabolic systems such as cell cycle regulation, several enzyme activities, DNA repair and transcription factor regulations [45]. In our present study, representing a severe lipid peroxidation state, GSH levels in APAP-treated AG group rats were significantly observed to be low when compared with the HG group (p<0.001). After treatment with DXP, GSH levels were increased significantly close to healthy group values in the ADXG and ANDXG groups both in hepatic and renal tissues (p<0.001). In renal tissues of the ANG group, a slight increase was observed in GSH levels, but this cannot reach any statistical significance when compared with the AG group (p>0.05).
SOD and CAT are enzymic antioxidants that protect the tissues against lipid peroxidation. CAT prevents hydroxyl radical generation, and protects the cellular composition from oxidative damage. Thus, the reduction of CAT activity may result in the accumulation of free oxygen radicals [46]. In some previous studies, it has been shown that DXP has antioxidative, anti-inflammatory properties and free radical scavenger effects [22–24]. CAT levels were significantly lower in the AG group compared with the HG group, emphasizing oxidative hepatorenal damage found in our study.
When compared with the HG group, hepatic and renal injury markers were significantly higher in the AG group. These markers showed a nearly 2- to 3-fold higher increase from baseline in the AG group. In some recent studies, different kinds of antioxidants like quercetin, curcumin, vitamins E and C were demonstrated to prevent the elevation of hepatorenal injury markers [34-37,47]. In accordance with these literature data, as an antioxidant, DXP prevented the elevation of hepatorenal function tests in the ADXG group in our study. These results emphasize the preventive antioxidant efficacy of DXP from the oxidative damage induced by APAP.
Our biochemical results were consistent with the histological findings, while oxidant parameters were found elevated in the APAP-treated group, thus indicating hepatorenal injury. Centrilobular hepatic necrosis, hemorrhage and sinusoidal dilatation, concomitant with inflammatory cells in hepatic sections, and tubular dilatation with damaged glomeruli and necrosis in kidney sections were observed in the histological assessment. The histologically preventive efficacy of DXP has been shown in ischemia-reperfusion-induced testicular oxidative damage and bleomycin-induced pulmonary fibrosis models of rats [48,49]. Correspondingly in our study, DXP was verified with nearly normal organ histologic structures except minimal hepatic sinusal dilatations and minimal renal focal tubular dilatations. While DXP and NAC therapy showed nearly the same histological improvement on hepatic sections, NAC alone appeared to be ineffective in renal protection with concomitant tubular dilatations and glomerular damage in renal sections. No additional benefit was observed when DXP and NAC were administered simultaneously.
In our study, no statistically significant difference occurred between the NAC-administered groups, DXP-administered groups and DXP+NAC simultaneously administered groups, in terms of oxidant and antioxidant parameters in hepatic tissues. DXP and NAC prevented liver damage nearly equally from APAP-induced oxidative stress. This means that DXP is as efficacious as the standard applicable antidote of APAP-induced hepatoxicity, but the administration of DXP and NAC together has no additive effect on the liver toxicity according to our data. However, in renal tissues, there was a statistically significant difference between the ANG and ADXG groups and the ANG and ANDXG groups in terms of oxidant/ antioxidant parameters (p<0.001), but there was no significant difference between the ADXG and ANDXG groups. These results indicated that NAC had no preventive effect on APAP-induced renal toxicity. Conversely, NAC was reported to be protective on APAP-induced nephrotoxicity in a few recent studies [27]. This difference between studies may depend on the NAC doses used. In the study of Kheradpezhoug et al. NAC doses used at least 200 mg/kg rising to 800 mg/kg in different rat groups. And in the 800 mg/kg NAC-used group, there was a better improvement in nephrotoxicity which was superior to the 200 mg/kg in the NAC group. We used 100 mg/kg NAC in the present study, and this dose may be insufficient to protect kidneys from APAP intoxication.
In summary, oxidative stress is one of the leading factors in APAP-induced toxicity. NAC, used as the known standard therapy in APAP toxicity, may not be effective in all cases as mentioned above. DXP has anti-inflammatory and antioxidant efficacy by increasing the levels of reduced GSH. Additionally, it has been shown in some previous studies that only CoA precursor products of PA may have these efficacies, indicating that the protective effect was due to this coenzyme. Apart from this direct protective effect of DXP via CoA against oxidative damage, CoA may also contribute to the prevention of cell damage by promoting cellular repair mechanisms [24]. Furthermore, in APAP toxicity, mitochondrial respiration is inhibited and cellular ATP contents are decreased [11]. It is known that DXP stimulates ATP synthesis in cells [20,21]. Probably, DXP may also prevent hepatorenal damage by supporting the mitochondrial bioenergetic system. Taking into consideration all the properties of DXP reveals that this safe, cost effective and readily available molecule has a key role in APAP toxicity. We think that DXP may be used in the daily clinical practice of acute hepatorenal APAP-induced toxicity, but further studies are needed to highlight its efficacy.
Funding
This study was supported by Adnan Menderes University Research Fund. Project Grant Number: 2015/15067636/604-01-46 TPF 15024
Conflict of Interest
The authors declare that they have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Chun LJ, Tong MJ, Busuttil RW, Hiatt JR. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol 2009; 43: 342-349.
- Bunchorntavakul C, Reddy KR. Acetaminophen-related hepatotoxicity. Clin Liver Dis 2013; 17: 587-607.
- Blakely P, McDonald BR. Acute renal failure due to acetaminophen ingestion: a case report and review of the literature. J Am Soc Nephro 1995; 6: 48-53.
- Moller-Hartmann W, Seigers CP. Nephrotoxcicity of paracetamole in the rat. Mechanistic and therapeutic aspects. J Appl Toxicol 1992; 11: 141-146.
- Mowry JB, Spyker DA, Brooks DE, McMillan N, Schauben JL. 2014 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol 2015
- Ostapowicz GA, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SBH, Lee WM. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; 137: 945-954.
- Larson AM, Polson J, Fontana RJ. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005; 42: 1364-1372.
- Mazer M, Perrone J. Acetaminophen-induced nephrotoxicity: pathophysiology, clinical manifestations, and management. J MedToxicol 2008; 4: 2-6.
- James LP, Mayeux PR, Hinson JA. Acethaminophen induced hepatotoxicity. Drug MetabDispos 2003; 31: 1499-1506.
- Hart SE, Beierschmitt WP, Wyand DS. Acetaminophen nephrotoxicity in CD-1 mice: I. Evidence of a role for in situ activation in selective covalent binding and toxicity. ToxicolApplPharmacol.1994; 126: 267-275.
- Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int 2012; 32: 8-20.
- Balantz RC. Acetaminophen: acute and chronic effects on renal function. Am Kidney Dis 1996; 28: 3-6.
- Uysal HB, Dagli B, Yilmaz M, Kahyaoğlu F, Gökçimen A, Ömürlü İK, Demirci B. Biochemical and Histological Effects of Thiamine Pyrophosphate against Acetaminophen-Induced Hepatotoxicity. Basic Clin Pharmacol Toxicol 2015.
- Abraham P. Vitamin C may be beneficial the prevention of paracetamol induced renal damage. Clin Exp Nephrol 2005; 9: 24-30.
- Abdel-Azeem AS, HegazyAM, Ibrahim KS, Farrag AR, El-Sayed EM. Hepatoprotective, antioxidant, and ameliorative effects of ginger (Zingiberofficinale Roscoe) and vitamin E in acetaminophen treated rats. J Diet Suppl 2013; 10:195-209.
- Slitt AL, Dominick PK, Roberts JC, Cohen SD. Standard of care may not protect against acetaminophen induced nephrotoxicity. Basic Clin Pharmacol Toxicol 2004; 95: 247-248.
- Kozer E, Koren G. Management of paracetamol overdose: current controversies. Drug Safety 2001; 24: 503-512.
- Abiko Y, Tomikawa M, Shimizu M. Enzymatic conversion of pantothenylalcohol to pantothenic acid. J Vitaminol 1969; 15: 59-69.
- Ebner F, Heller A, Rippke F, Tausch I. Topical use of dexpanthenol in skin disorders. Am J ClinDermatol 2002; 3: 427-433.
- Slyshenkov VS, Piwocka K, Sikora E, Wojtczak L. Pantothenic acid protects jurkat cells against ultraviolet light-induced apoptosis. Free RadicBiol Med 2001; 30: 1303-1310.
- Slyshenkov VS, Dymkowska D, Wojtczak L. Pantothenic acid and pantothenol increase biosynthesis of glutathione by boosting cell energetics. FEBS Lett 2004; 569: 169-172.
- Slyshenkov VS, Omelyanchik SN, Moiseenok AG, Trebukhina RV, Wojtczak L. Pantothenol protects rats against some deleterious effects of gamma radiation. Free Radic Biol Med 1998; 24: 894-899.
- Wojtczak L, Slyshenkov VS. Protection by pantothenic acid against apoptosis and cell damage by oxygen free radicals-the role of glutathione. BioFactors 2003; 17: 61-73.
- Slyshenkov VS, Rakowska M, Moiseenok AG, Wojtczak L. Pantothenic acid and its derivatives protect Ehrlich ascites tumor cells against lipid peroxidation. Free Radic Biol Med 1995; 19: 767-772.
- McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol 2012; 264: 387-394.
- Elshazly SM, El-Moselhy MA, Barakat W. Insights in the mechanism underlying the protective effect of α-lipoic acid against acetaminophen-hepatotoxicity. Eur J Pharmacol 2014; 726: 116-123.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351-358.
- Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem 1983; 132: 345-352.
- Beutler E, Durgun O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963; 51: 882-888.
- Catalase AH. Methods of enzymatic analysis. Academic Press, New York, 1974.
- Navarro-Gonzalves JA, Garcia-Benayas C, Arenas J. Semiautomated measurement of nitrate in biological fluids. Clin Chem 1998; 44: 679-681.
- Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanism, analogues, and protective approaches. Rev Toxicol 2001; 31: 55-138.
- Kisaoglu A, Borekci B, Yapca OE, Bilen H, Suleyman H. Tissue damage and oxidant/antioxidant balance. Eurasian J Med 2013; 45: 47-49.
- Kheradpezhouh E, Panjehshahin MR, Miri R, Javidnia K, Noorafshan A, Monabati A, Dehpour AR. Curcumin protects rats against acetaminophen-induced hepatorenal damages and shows synergistic activity with N-acetyl cysteine. Eur J Pharmacol 2010; 628: 274-281.
- Kannan N, Sakthivel KM, Guruvayoorappan C. Protective effect of Acacia nilotica against acethaminophen-induced hepatocellular damage in Wistar rats. Adv Pharmacol Sci 2013; 2013: 987692.
- Cekmen M, Ilbey YO, Ozbek E, Simsek A, Somay A, Ersoz C. Curcumin prevents oxidative renal damage induced by acetaminophen in rats. Food ChemToxicol. 2009; 47: 1480-1484.
- Ahmad ST, Arjumand W, Nafees S, Seth A, Ali N, Rashid S, Sultana S. Hesperidin alleviates acetaminophen induced toxicity in wistar rats by abrogation of oxidative stress, apoptosis and inflammation, Toxicol Lett 2012; 208: 149-161.
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 2002; 82: 47-95.
- Ximenes VF, Paino IM, Faria-Oliveira OM, Fonseca LM, Brunetti IL. Indole ring oxidation by activated leukocytes prevents the production of hypochlorous acid. Braz J Med Biol Res 2005; 38: 1575-1583.
- Kumerova AO, Silova AA, Utno LY. Effect of pantethine on postheparinlipolytic activity and lipid peroxidation in the myocardium. Biull Eksp Biol Med 1991; 111: 33-35.
- Davenport A, Finn R. Paracetamol (acetaminophen) poisoning resulting in renal failure without hepatic coma. Nephron.1988; 50: 55-56.
- Kapp A, Zeck-Kapp G. Effect of Ca-pantothenate on human granulocyte oxidative metabolism. Allerg Immunol 1991; 37: 145-150.
- Gardner CR, Heck DE, Yang CS, Thomas PE, Zhang XJ, DeGeorge GL. Role of nitric oxide in acetaminophen-induced hepatotoxicity in the rat. Hepatology 1998; 27: 748-754.
- Hayes JD, McLellan LI. Glutathione and glutathione dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res 1999; 31: 273-300.
- Fernandez-Checa JC, Garcia-Ruiz C, Colell A, Morales A, Mari M, Miranda M, Ardite E. Oxidative stress: role of mitochondria and protection by glutathione. Biofactors 1998; 8: 7-11.
- Scott MD, Lubin BH, Zuo L, Kuypers FA. Erythrocyte defense against hydrogen peroxide: preeminent importance of catalase. J Lab Clin Med. 1991; 118: 7-16.
- El-Shafey MM, Abd-Allah GM, Mohamadin AM, Harisa GI, Mariee AD. Quercetin protects against acetaminophen-induced hepatorenal toxicity by reducing reactive oxygen and nitrogen species. Pathophysiology 2015; 22: 49-55.
- Etensel B, Ozkisacik S, Ozkara E, Karul A, Oztan O, Yazici M, Gürsoy H. Dexpanthenol attenuates lipid peroxidation and testicular damage at experimental ischemia and reperfusion injury. Pediatr Surg Int 2007; 23: 177-181.
- Ermis H, Parlakpinar H, Gulbas G, Vardi N, Polat A, Cetin A, Kilic T, Aytemur ZA. Protective effect of dexpanthenol on bleomycin-induced pulmonary fibrosis in rats. Naunyn Schmiedebergs Arch Pharmacol 2013; 386: 1103-1110.