ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2022) Volume 33, Issue 5
Protective effects of Vitis vinifera seed extract on hepatotoxicity induced by anabolic androgenic steroids in male mice.
Hani M. Abdelsalam*
Department of Zoology, Zagazig University, Zagazig, Egypt
Accepted date: July 12, 2022
Background: Anabolic androgenic steroids are synthetic derivatives of the male sex hormone Testosterone (TE). Androgens and anabolic steroids have been used for therapeutic purposes and have been widely used among adolescents for body-building purposes, with no consideration as to its adverse effects. The aim of this study was to assess the effect of exposure to a high dose of testosterone on the liver and assess the hepatoprotective potency of the Vitis vinifera (VT) seed extract against testosterone-induced hepatotoxicity.
Methods: Thirty-two male BALB/C mice were assigned to four groups: control, TE, VT, and TE+VT. Liver functions tests, lipid profile, and antioxidants parameters were evaluated to assess hepatotoxicity and histopathological analysis was performed to explain the direct effects of TE and VT on hepatic tissue.
Results: The high dose of testosterone exhibited serious hepatotoxic effects, with disturbance of the lipid profile (total cholesterol, triglycerides, low-density lipoprotein, and high-density lipoprotein) and antioxidative balance. The high content of flavonoids and phenolic compounds in the VT seeds demonstrated excellent antioxidant and antihepatotoxic activity. More importantly, a histopathological analysis confirmed the defensive role of VT for hepatocytes and all hepatic constituents against the toxic storm caused by testosterone.
Conclusion: Accordingly, VT demonstrated protective and restorative effects against testosterone hepatotoxicity and could be a promising new antihepatotoxic agent. Therefore, it is recommended that VT should be administered during the use of or treatment with testosterone.
Keywords
Vitis vinifera, Anabolic androgenic steroids, Testosterone, Biomarkers, Hepatocytes.
Abbreviations
TE: Testosterone; VT: Vitis vinifera; TC: Total Cholesterol; LDL: Low-Density Lipoprotein; HDL: High-Density Lipoprotein; MDA: Malondialdehyde; SOD: Superoxide Dismutase; GSH: Glutathione; ROS: Reactive Oxygen Species.
Introduction
Some adolescents and young adults, most of whom are male [1], combine endurance-oriented physical training with the use of testosterone and/or its synthetic derivatives known as Anabolic Androgenic Steroids (AAS). The main goal is to reduce body fat faster and boost muscle building with the aim of improving physical performance and appearance [2] thereby contributing to self-esteem.
The abuse of AAS and the prototypical testosterone is associated with a variety of severe adverse health effects [3], including several directly linked to liver injury [4]. A growing body of data links this damage to a variety of genomic and non-genomic actions caused by these drugs, including enhanced generation of reactive oxygen species and lipid peroxidation and the related activation of cellular stress-signalling pathways [5]. Disruption of redox homeostasis is a well-established event in drug hepatotoxicity and various liver diseases [6]. The primary sources of reactive oxygen species, the noxious mediators of oxidative stress, are cytochrome P450 enzymes of the endoplasmic reticulum and mitochondria [7] and several enzymes that are present in the abundant peroxisomes in the liver [8].
Grape seed extract contains most of the active compounds of black grapes (Vitis vinifera), which is one of the most widely grown fruit crops in the world [9]. Owing to its high levels of antioxidants and polyphenols, this extract has gained the interest of scientists and has shown promising anti-inflammatory and immunomodulatory [10], antioxidant [11], anti-diabetes and anti-atherosclerotic [12], antiaging [13], and anticancer properties [14]. Resveratrol is a grape seed phytoalexin extract, whose production peaks in response to environmental stresses such as plant infections, climate, ozone, and ultraviolet light [15]. Grape extracts are rich in polyphenol compounds with significant antioxidant activity and have protective effects against oxidative damage in the liver [16]. These compounds play a pivotal role in the protective and therapeutic actions of grape seed extracts.
Materials and Methods
Ethics statement
All animal protocols were approved by the Ethical Committee of Zagazig University (ZU-IACUC committee)
Experimental animals
Thirty-two healthy adult male BALB/C mice weighing 32- 38 g were obtained and maintained at the Breeding Animal House of the Faculty of Medicine, Zagazig University, Egypt. The mice were acclimatized for 1 week in plastic cages with stainless steel wire-bar lids at a controlled temperature (23°C ± 1°C) and humidity (55% ± 5%) in a 12:12 h light-dark cycle in an artificially illuminated room, completely free from chemical contamination. The mice were fed and watered ad libitum. To provide humane care in compliance with the guidelines of the Ethics Committee of Zagazig University (ZU-IACUC committee) and in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, all mice were treated by the head of the Zoology Department, Faculty of Science, Zagazig University. The reporting in the manuscript follows the recommendations in the ARRIVE guidelines.
Experimental design
The animals were assigned to the following groups
Control group: This group included eight mice that were administered sesame oil (Sigma–Aldrich, St. Louis, MO, USA) for 6 weeks.
TE group: This group included eight mice that were administered testosterone injected intramuscularly (Sigma-Aldrich, St. Louis, MO, USA) mixed with sesame oil as necessary to provide the same injection volume of 1 mL/kg of body weight, irrespective of the weekly testosterone dose employed. Testosterone was injected at dosage of 80 mg/kg/week (each Sunday) for six weeks, alternatively into the left and right hind legs [17].
VT group: This group included eight mice that were administered an aqueous VT extract (400 mg/kg) daily by oral gavage for 6 weeks [11].
TE+VT group: This group included eight mice that were administered testosterone injected intramuscularly (as in the TE group) with the simultaneous administration of VT (as in the VT group).
At the end of the experiment, all of the mice were anesthetized with intraperitoneal injections of 25 mg/kg sodium thiopental [18]. Blood samples were collected [19] for biochemical analysis, and the mice were euthanized to obtain their livers for histological study.
Experimental Procedures
Plant source
The ripe VT fruits were collected from June to July 2021 from the fruit market sector (Zagazig, Sharkia governorate, Egypt) and were authenticated through chemical tests and deposited and deposited in the Pharmacology Department (Faculty of Pharmacy, Zagazig University).
Preparation of aqueous extracts from Vitis vinifera fruits
The VT seeds were separated from the pulp manually and shade dried (25°C-30°C). The dried seeds were ground to powder and soaked for 24 h in an appropriate volume of Millipore distilled water to obtain a 200-mg/mL solution, which was then filtered through 0.22 μm sterile filters with the help of a vacuum pump. The resulting solution was then stored in a brown bottle at 4°C until use [11].
Blood sample collection
Blood samples were collected from the retro-orbital venous plexus, under mild anesthesia, using a fine heparinized capillary tube introduced into the medial epicanthus of the mouse’s eye [19]. The blood samples were collected in a clean graduated centrifuge tube, allowed to clot at room temperature for 10 min, and then centrifuged using a Remi cooling centrifuge at 5000 rpm for 20 min. The supernatant serum was collected in a clean dry tube for the biochemical tests. All the biochemical procedures were conducted in the Central Research Laboratory (Faculty of Medicine, Zagazig University).
Biochemical estimation of blood parameters
Serum Alanine Aminotransferase (ALT), Alkaline Phosphatase (ALP) and Aspartate Aminotransferase (AST) activity was measured by colorimetry [20] using assay kits (Sigma Chemical Corp., St Louis, MO, USA) using an automated analyzer method (Bayer Autoanalyzer). Serum total protein [21] and total bilirubin levels were determined according to the Doumas et al., method [22].
Biochemical estimation of serum lipid profile
Triglycerides, total cholesterol, High Density Lipoprotein (HDL) cholesterol and Low Density Lipoprotein (LDL) cholesterol concentrations were evaluated enzymatically using assay kits (Sigma Chemical Corp., St. Louis, MO, USA). Very LDL (VLDL) cholesterol levels were calculated as triglycerides, and LDL cholesterol levels were calculated using the following equation: LDL cholesterol = total serum cholesterol-(HDL+VLDL) [23].
Lipid peroxidation estimation (as reflected in the amounts of measurable malondialdehyde)
Lipid peroxidation was estimated by measuring the level of thiobarbituric acid reactive substances in tissues using the Niehaus and Samuelsson method [24]. Briefly, the tritiated acid was treated with diazomethane, diluted with methyl arachidonate, and purified by chromatography on a column of silicic acid impregnated with silver nitrate. The product was radiochemically pure as judged by thin-layer chromatography and gas-liquid chromatography and had a specific activity of 5 × l09 disintegrations/min/μmol.
Superoxide dismutase estimation
Superoxide Dismutase (SOD) was assayed using the Lima et al. method [25]. Briefly, the assay mixture in a total volume of 1 mL consisted of 0.1 mol/L of sodium phosphate buffer (pH 7.8) and 0.08 mmol/L of Ethylenediamine Tetraacetic Acid (EDTA) at a 1:1 proportion. After dilution, 0.1-mL of the tissue sample (1:1000) was added to 2.3 mL of distilled water, after which 1 mL of assay mixture with EDTA and sodium phosphate buffer was added. The increase in absorbance due to quercetin oxidation at 0 and 20 min was measured spectrophotometrically at 406 nm. In the blank, a tissue sample was substituted by equal quantities of distilled water. One unit of SOD activity was defined as the quantity of the enzyme that inhibited quercetin oxidation by 50% under given experimental conditions.
Glutathione estimation
Glutathione (GSH) concentrations were determined using the Niehaus and Samuelsson method [24]. Briefly, reduced GSH was measured by reaction with 5,5'-dithiobis(2- nitrobenzoic acid) to yield a compound that absorbs at 412 nm (Ellman’s method). Reduced GSH in the supernatant fractions was also assayed enzymatically using GSH S-transferase.
Catalase activity estimation
Catalase Activity (CAT) was estimated using the Luck method [26]. The ultraviolet absorption of hydrogen peroxide can be measured at 240 nm, and its absorbance drops when degraded by the enzyme catalase. The enzyme activity can be calculated from the decrease in absorbance.
Histopathological study
All steps were conducted in the Histology and Cell Biology Department (Faculty of Medicine, Zagazig University). Specimens from the liver and renal cortex of each animal were fixed in 10% neutral formal saline, dehydrated, embedded in paraffin wax, and processed into 5 μm thick sections that were then stained with hematoxylin and eosin. The stained slides were analyzed by light microscopy in the Image Analysis Unit [27].
Statistical analysis
The data are presented as mean ± standard deviation. The statistical analysis was performed using the Statistical Package for the Social Sciences version 13.00 (Chicago, IL, USA). Statistical significance was applied by a oneway analysis of variance for differences between the means of the study groups. Probability values P< 0.001 reflected statistical significance.
Results
Biochemical results
The TE group exhibited significantly higher mean total bilirubin, ALT, AST, and ALP levels than the control group (P<0.001), while total protein levels significantly decreased. The VT group showed significantly lower mean ALT, AST, and ALP levels than the control group (P<0.001). The TE+VT group showed a significant reduction in total bilirubin, ALT, AST, and ALP levels compared with the TE group (P<0.001). The serum parameter results for the study groups are presented in Table 1.
| Group | ||||
|---|---|---|---|---|
| Control | TE | VT | TE + VT | |
| TP (g/dL) | 5.30 ± 0.41 | 3.18 ± 0.58a | 6.69 ± 0.46ab | 4.56 ± 0.81b |
| TB (mg/dL) | 0.45 ± 0.03 | 0.86 ± 0.05a | 0.35 ± 0.05b | 0.63 ± 0.06ab |
| ALT (U/L) | 19.75 ± 0.85 | 48.25 ± 1.75a | 17.51 ± 0.61ab | 25.72 ± 169ab |
| AST (U/L) | 63.91 ± 2.61 | 94.49 ± 2.85a | 57.72 ± 1.83ab | 83.49 ± 1.52ab |
| ALP (U/L) | 170.9 ± 7.54 | 231.6 ± 7.94a | 147.5 ± 7.34ab | 175.0 ± 7.01b |
Table 1. Serum parameters for the study groups.
ALP: Alkaline Phosphatase; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; TB: Total Bilirubin; TE: Testosterone; TP: Total Protein; VT: Vitis vinifera
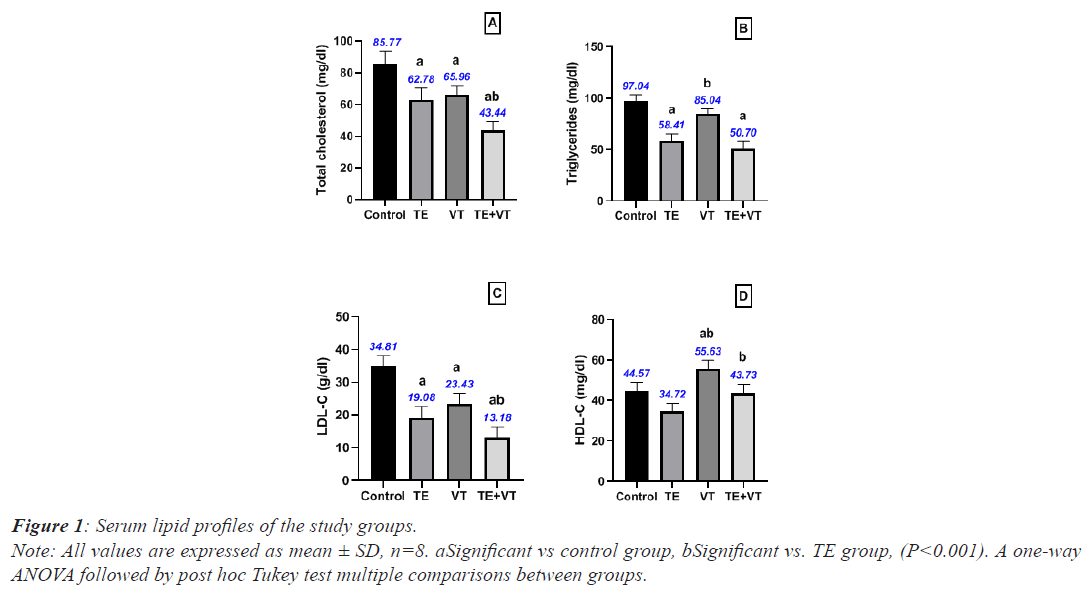
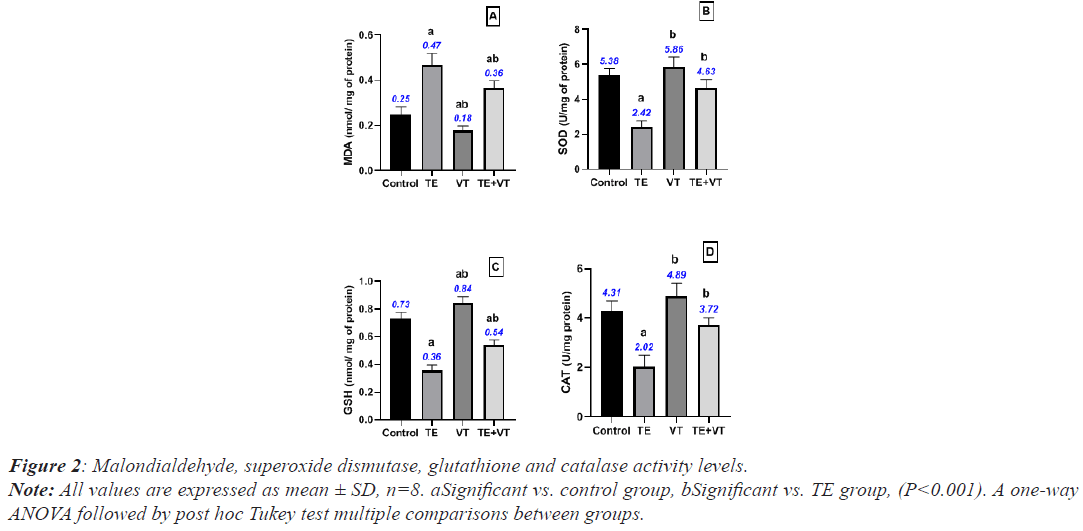
Note: All values are expressed as mean ± SD, n=8. aSignificant vs. control group, bSignificant vs. TE group (P<0.001). A one-way ANOVA followed by post hoc Tukey test multiple comparisons between groups.
The TE group presented significantly lower mean Total Cholesterol (TC), triglycerides, and LDL levels compared with the control group (P<0.001), while the VT group showed significantly lower mean TC and HDL levels than the control group (P<0.001). Moreover, the TE+VT group showed a significant reduction in TC and LDL levels, while HDL levels significantly increased compared with the TE group (P<0.001). The statistical results of the study groups are shown in Figure 1.
The TE group presented significantly higher mean SOD, GSH, and CAT levels than the control group (P<0.001), while MDA increased significantly. The VT group present significantly higher mean SOD, GSH, and CAT levels than the control group (P<0.001). The TE+VT group showed a significant reduction in SOD, GSH, and CAT levels, while MDA levels showed a significant increase compared with the TE group (P<0.001). The statistical results of the study groups are presented in Figure 2.
Histopathological results
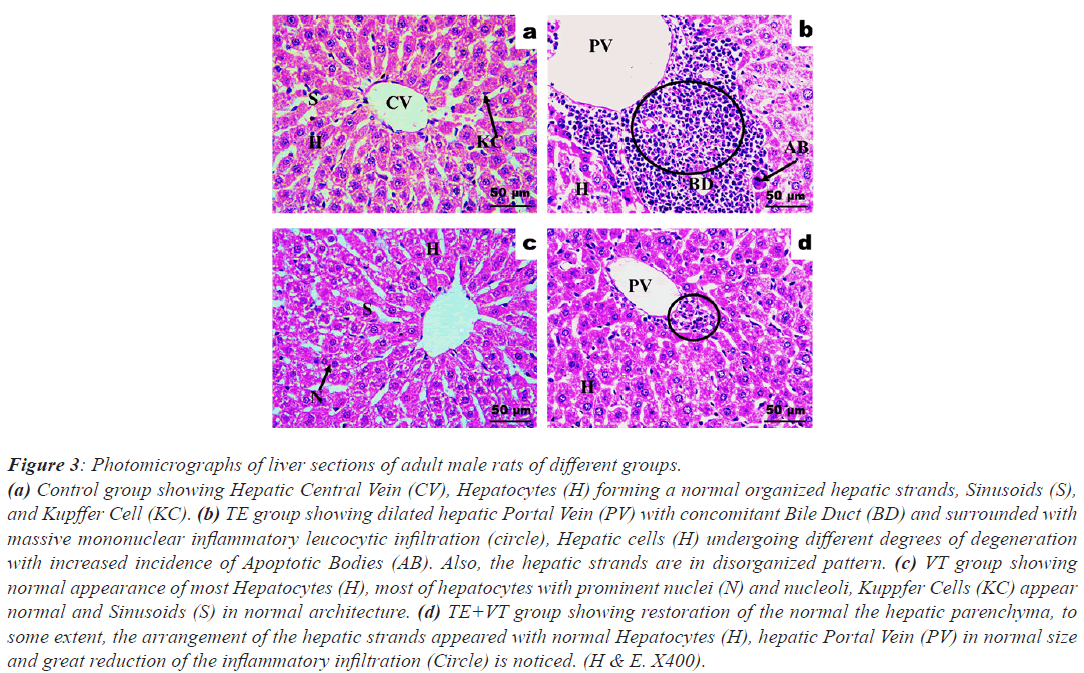
Figure 3 shows photomicrographs of the liver sections from each study group. The control group shows normal organized hepatic strands, sinusoids, and Kupffer cell. The TE group shows a dilated hepatic portal vein, with concomitant bile duct, surrounded by massive mononuclear inflammatory leucocytic infiltration, with hepatic cells undergoing varying degrees of degeneration with an increased incidence of apoptotic bodies. The hepatic strands are in a disorganized pattern. The VT group shows normal looking hepatocytes with prominent nuclei and nucleoli, Kuppfer cells, and sinusoids. The TE+VT group shows restoration of the normal hepatic parenchyma, arranged hepatic strands with normal hepatocytes, normal hepatic portal vein, and a major reduction in inflammatory infiltration.
Figure 3: Photomicrographs of liver sections of adult male rats of different groups.
(a) Control group showing Hepatic Central Vein (CV), Hepatocytes (H) forming a normal organized hepatic strands, Sinusoids (S),
and Kupffer Cell (KC). (b) TE group showing dilated hepatic Portal Vein (PV) with concomitant Bile Duct (BD) and surrounded with
massive mononuclear inflammatory leucocytic infiltration (circle), Hepatic cells (H) undergoing different degrees of degeneration
with increased incidence of Apoptotic Bodies (AB). Also, the hepatic strands are in disorganized pattern. (c) VT group showing
normal appearance of most Hepatocytes (H), most of hepatocytes with prominent nuclei (N) and nucleoli, Kuppfer Cells (KC) appear
normal and Sinusoids (S) in normal architecture. (d) TE+VT group showing restoration of the normal the hepatic parenchyma, to
some extent, the arrangement of the hepatic strands appeared with normal Hepatocytes (H), hepatic Portal Vein (PV) in normal size
and great reduction of the inflammatory infiltration (Circle) is noticed. (H & E. X400).
Discussion
AAS is an important hormone that participates in a variety of physiological functions, including embryonic development [28], stimulation of genital growth and secondary sexual characteristics [29], and promotion of erythropoiesis [30]. Elevated serum AST, ALT, ALP, and gamma-glutamyl transferase levels are well-known markers of AAS hepatotoxicity [31]. In humans, AAS levels are usually elevated in liver diseases involving cholestasis and jaundice, including those caused by AAS [32]. In rats, AAS is supposedly a better cholestasis marker than serum ALP but is less reliable than in other species [33].
This study’s main finding is that testosterone supplementation enhanced hepatic stress by significantly increasing total bilirubin, ALT, AST, and ALP levels, while significantly reducing total protein levels. This result is consistent with a study performed by Kahal et al. [34] who showed an increase in plasma levels of liver enzymes (ALT and AST) over the course of AAS treatment, accompanied by significant degenerative changes in hepatic tissue after two months of treatment. The elevation of AST and ALT levels might be attributed to hepatic disorder in the form of hepatitis and cholestasis induced by testosterone abuse [35]. Dickerman et al. [36] reported that the elevation of hepatic enzymes could be related to the suppression of the hypothalamic-pituitary-testicular axis. Heart damage could be considered another reason for increased AST levels in treated rats compared with control rats. A study by Alen et al. reported that ASS can cause AST leakage from muscle to serum in athletes [37].
In the present study, an orally administered seed extract of VT prevented hepatocyte destruction and recovered hepatocytes, as evidenced by the near normal serum levels of ALT, AST, ALP, and total bilirubin. Serum total protein was also maintained at near normal levels following supplementation with the seed extract. These results are supported by Almajwal and Elsadek [38] who indicated that rats that consumed diets containing ground Vitis seeds (5%, 10%, and 20%) significantly attenuated the elevated levels of serum hepatic markers, suggesting that Vitis seeds can protect cell membrane integrity against paracetamolinduced leakage of hepatic marker enzymes into the blood circulation. Several studies have found that the bioactive compounds in grape seed, such as proanthocyanin, could protect the liver against ethanol-induced inflammation in normal, nondiabetic conditions in rats [39]. Other studies have indicated that the pro-anthocyanin in grapes could generally protect the liver against carbon tetrachlorideinduced fibrosis and inflammation in non-diabetic conditions [40].
Testosterone shifts the lipid profile, where it is usually linked to reduced atherogenicity in humans [41] and rats [42]. This study has shown that testosterone has lipolysis effects where testosterone greatly reduced all lipid profile parameters (Total Cholesterol (TC), triglycerides, LDL, and HDL). The low cholesterol concentration in the treated mice was in agreement with Whitsel et al. [43] who stated that this reduction could be attributed to the upregulation of Scavenger Receptor B1 (SR-B1) in hepatocytes and macrophages and thereby stimulates the selective cholesterol uptake and efflux respectively [44]. Decreased HDL-C levels in the treated male mice agree with the results by Whitsel et al. [43] and could result from upregulated testosterone to the two genes involved in HDL-C catabolism, namely hepatic lipase and SR-B1, the latter of which mediates the selective uptake of HDL-C lipids into hepatocytes and steroidogenic cells [45]. The reduction in LDL-C levels in response to testosterone treatment was in agreement with Whitsel et al. [43]. The decreased LDL-C levels in the treated animals might be due to the increase in LDL-C oxidation induced by the increased Reactive Oxygen Species (ROS) production and increased lipid peroxidation in response to testosterone injection [46]. Another cause for the low LDL-C levels in response to testosterone injection might be related to the disturbance of the regulatory mechanism of LDL receptor function induced by testosterone as a result of the cells possessing a high number of LDL receptors with high affinity even to oxidized LDL-C, as occur in macrophages found in atherosclerotic plaque, which might be found in testosterone-treated rats [46]. An open-label, cross-over study reported that the transdermal administration of testosterone enhanced fat oxidation, suggesting a direct regulation of hepatic fat oxidation by testosterone [47].
According to the present results, Vitis seeds have a lipid-lowering effect on TC, triglycerides, and LDL while causing an increase in HDL levels in the VT and TE+VT groups. Studies have shown that the flavonoids and phenolic compounds of Vitis seeds can inhibit oxidation processes and act cytotoxically, resulting in the development of various diseases [48], and can increase HDL-C concentrations and decrease LDL and VLDL levels in hypercholesteremic and hepatotoxic rats [49].
The levels of MDA, lipid peroxide, GSH, GSH peroxidase, CAT, and SOD in hepatic tissues represent the state of oxidative stress [50]. Decreased activity of liver GSH, CAT, and SOD in high dose testosterone-treated rats and the negative correlations between these enzyme activities and weekly testosterone doses indicated the damaging action of massive testosterone supplementation on liver antioxidant enzymes [17]. The present study showed that testosterone significantly lowered antioxidant levels (SOD, GSH, and CAT) and increased MDA levels, while treatment with Vitis seeds reversed these results. ROS, the unstable and extremely reactive by-products of normal metabolism, mediate oxidative damage to cellular macromolecules [51]. Given that testosterone typically increases the metabolic rate [52], a high dose of testosterone can be expected to be involved in the imbalance between ROS production and antioxidant defenses, causing an increased risk of oxidative stress. Several researchers have reported that testosterone plays a pro-oxidant role and induces oxidative stress in mammalian tissues [52]. Chainy et al. [53] reported elevated MDA levels in response to testosterone treatment. Testosterone injection in our study caused a reduction in antioxidant enzymes (GSH and SOD), which agrees with findings reported by Chainy et al. [53]. A reduction in SOD activity causes an increase in oxygen levels, which in turn causes inactivation of CAT activity. Similarly, when GSH or CAT fails to eradicate hydrogen peroxide, the latter can be upregulated by SOD inactivation and vice versa [53]. In general, the administration of exogenous testosterone causes antioxidant enzyme reduction followed by oxidative stress induction. Phytochemical screening showed that the Vitis seed extract contains flavonoids and phenols, the two compounds known to control antioxidant activity [54]. Therefore, the VT seed extract helped reduce oxidative stress in the livers of the TE group by eliminating the free radicals. The increased MDA content might have resulted from an increase in ROS as a result of the stress condition in the mice after the testosterone injection.
The abnormal liver function test parameters in the serum of the TE group indicative of hepatocellular damage were supported by the histopathological changes in the liver, as revealed by the moderate to severe necrosis. The structural changes might cause disorganization of the hepatic architecture and inflammatory infiltration, resulting in increased serum levels of AST, ALP, ALT, and total bilirubin in the TE group. This alteration might have been caused by the oxidative stress resulted from the testosterone treatment [52]. The antioxidant activities of the VT seed [54] prevented the histopathological changes in the liver and lowered the serum levels of liver enzymes and total bilirubin. Lesser signs of necrosis and connective tissue inflammation were observed in the liver of the VTtreated TE group.
Conclusion
VT demonstrated protective and restorative effects against testosterone hepatotoxicity biomarkers and the hepatic histology. In addition, this study demonstrated the antioxidant effects of VT; thus it ameliorated the inflammatory and oxidative effects caused by testosterone. Therefore, it is recommended that VT should be administered during treatment with testosterone, but with strictly observations for the other systems such as GIT, CVS, and hepatic system, so further studies are required to explain the effect of VT and testosterone on these systems.
Acknowledgement
I am grateful to those who instilled in me the value of education and the rewards and opportunities it can generate for my parents, especially my father, who supplied me with enthusiasm, support, and creative insight. His critical reading of the manuscript helped me refine the concept of this thesis; his deep interest in the topic, and unfailing encouragement are highly appreciated.
Author’s contribution
HA conducted the physiological, biochemical, and anatomical studies, participated in the sequence alignment, and drafted the manuscript.
Funding
The author did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors for this study.
Availability of Data Materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All animal protocols were approved by the Ethical Committee of Zagazig University (ZU-IACUC committee).
Consent for Publication
Not applicable.
Competing Interests
The author declares that there are no conflicts of interest.