ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2016) Volume 27, Issue 3
Protective effects of simvastatin, Nigella sativa oil and thmoquinone against dimethylnitrosamine-induced oxidative stress in rat kidney.
Edibe Saricicek1*, Ahmet Celik2, Nuray Uremis2, Metin Kilinc2
1Department of Medical Biochemistry, Medical Faculty, Zirve University, Gaziantep/ Turkey
2Department of Medical Biochemistry, Medical Faculty, Kahramanmaras Sutcu Imam University, Kahramanmaras/Turkey
- *Corresponding Author:
- Edibe Saricicek
Department of Medical Biochemistry
Medical Faculty, Zirve University
Turkey
Accepted Date: March 31, 2016
Background: Dimethylnitrosamine (DMN) is a potent member of nitrosamine family, which has toxic and carcinogenic potential. Several beneficial effects of statins have been described in patients with chronic kidney disease and in experimental models. The aim of this study is to investigate the protective effects of Simvastatin (SMN), Nigella Sativa Oil (NSO) and Thymoquinone (TQ) on DMN-induced nephrotoxicity in rats.
Methods: The animals were divided into five groups as: DMN Group, DMN+NSO Group, DMN+TQ Group and Control Group. Total antioxidant status (TAS), total oxidant status (TOS), Lipid hydroperoxide (LOOH) levels, and Total Thiol concentrations were measured.
Results: There was a remarkable difference between groups in terms of TAS, OSI, Total Thiol and LOOH concentrations (p values were <0,001; 0,014; <0,001 and 0,003 respectively) and was no remarkable difference between groups in terms of TOS (p=0,575). LOOH was significantly lower in DMN+NSO group than in DMN Group (p=0,046). None of the parameters were found significantly different between DMN Group and DMN+TQ Group. TAS and total thiol concentrations were significantly higher while OSI and LOOH were found significantly lower in DMN+SMN group than in DMN Group.
Conclusions: SMN prevented the kidney damage via its antioxidant effect whereas NSO had partial effect. But TQ did not have a remarkable protective effect.
Keywords
Dimethylnitrosamine, Oxidative stress, Simvastatin, Nigella sativa oil, Thymoquinone.
Introduction
While several reasons play role in kidney damage, radiocontrast agents, nephrotoxins (toxic agents and drugs) and ischemia are also blamed. Dimethylnitrosamine (DMN) is a potent member of nitrosamine family [1]. While the liver is the primary target for DMN metabolism, kidney, lung and esophagus are also targets [2]. The damage with DMN in tissues is not only via direct effect of reactive metabolites but also via production of proinflammatory agents such as cytolytic and reactive oxygen species or membrane lipid peroxidants. It has hepatotoxic and carcinogenic potential [1,2].
Nigella sativa (NS), also known as black seed or black cumin, has been used in folk medicine for a long time. NS contains more than 30% of a fixed oil and 0.40-0.45 w/w of a volatile oil. The volatile oil has been shown to contain 18-24% thymoquinone (TQ) and 46% monoterpenes such as p-cymene and a-pinene [3]. In recent clinical and experimental studies it has been reported that NS has immunomodulative, antiinflammatory, antitumour, antidiabetic and antiulcerogenic effects.
In addition, modern toxicological studies have shown that NS and some of its active components (volatile oil and TQ) may play a protective role on the disease or chemical induced nephrotoxicity and hepatotoxicity [4]. Statins are competitive inhibitors of 3-hydroxy-3-methyl- glutaryl-coenzyme A (HMG-CoA) reductase, the key enzyme that regulates synthesis of cholesterol from mevalonic acid by suppressing the conversion of HMG-CoA. Simvastatin is one of the antioxidative drugs, which has anti-inflammatory, antioxidant, antiapoptotic and anabolic effects [5].
In addition, several beneficial effects of statins have been described in experimental models of renal disease, including streptozotocin-induced diabetic nephropathy, puromycininduced nephrosis, and cyclosporin A (CsA)-induced nephropathy and in patients with chronic kidney disease [6-14]. This study aimed to investigate the protective effects of Nigella Sativa Oil (NSO), Thymoquinone (TQ) and Simvastatin (SMN) on DMN-induced oxidative stress in rat kidneys.
Materials and Methods
This experimental study was performed subsequent to the approval of the Institutional Animal Care and Use Committee of Kahramanmaras Sutcu ?mam University, Medical Faculty (05.05.2015/03-04).
Animal models
In this study, we used 30 male Wistar-Albino rats whose weights ranged from 200-250 grams. These animals were kept at constant temperature (20-220C) and humidity (50-60%) with a diurnal cycle of 12-hour light and 12-hour darkness periods. Animals were obtained from the facilities of the experimental research laboratory of our institution and access to food and water was allowed ad libitum. Procedures were carried out in accordance with the guidelines of the National Institute of Health guide for the care and use of laboratory animals (NIH Publication No. 8023, revised 1978). Maximum effort was spent for minimization of the suffering of animals as well as the reduction of the number of animals used. Before the beginning of the study, rats with unsuitable health conditions were removed from the main population.
Assignment of 30 male Wistar rats (weighing 200-250 g) was made into one control group (n=10), and two study groups (n=10 for each).
Preparation of the gavage solution
Three tablets of Simvastatin were diluted in 10 mL water, and then 2 mL from that was diluted in 10 mL water.
Induction of renal toxicity
Renal toxicity was induced in Wistar-Albino rats using a single intraperitoneal injection of 40 mg/kg DMN obtained from Sigma made up in normal saline.
Experimental design
The animals were divided into the following groups:
Control Group: The rats received standard food and water (no DMN, no NSO, no TQ and no SMN).
DMN Group: A single intraperitoneal injection of 40mg/kg DMN in normal saline.
DMN+NSO Group: A single intraperitoneal injection of 40mg/kg DMN in normal saline, then 2 mL/kg oral yolla 10gün per oral for ten days
DMN+TQ Group: A single intraperitoneal injection of 40 mg/kg DMN in normal saline, then 20 mg/kg oral yolla 21gün. per oral for 21 days
DMN+SMN Group: A single intraperitoneal injection of 40 mg/kg DMN in normal saline, then 10 mg/kg/day oral yolla per oral SMN for 12 days.
After 12 days from beginning of the study animals were sacrificed and kidneys were dissected from all groups at 4°C and quickly frozen in liquid nitrogen. Tissues were homogenized with suitable buffer, centrifuged at 3000 g and the supernatant were used for biochemical estimations on the same day of sacrifice.
Tissue extracts were stored at -70°C until further analysis.
Preparation of renal tissue homogenate
100 mg tissue was rinsed with 1X phosphate buffered solution (PBS) homogenized in 1 mL of 1X PBS and stored overnight at -20 degrees. After two freeze-thaw cycles were performed to break the cell membranes, the homogenates were centrifuged for 5 minutes at 5000xg, 2-8 degrees. The supinate was assayed and removed immediately. Alternatively, aliquots were stored at -80 degrees.
Measurement TAS, TOS and OSI
Total antioxidant status (TAS) and total oxidant status (TOS) were measured using a colorimetric method that was introduced by Erel [15,16]. The results were expressed as millimolar Trolox equivalent per liter (mmol Trolox equivalent/g protein) for TAS and micromolar hydrogen peroxide equivalent per liter (μmol H2O2 equivalent/g protein) for TOS. Oxidative stress index (OSI) value was calculated according to the following formula. OSI (arbitrary unit)=[TOS (μmol H2O2 equivalent/g protein)/TAS (μmol Trolox equivalent/g protein)]×100
Measurement Lipid hydroperoxide (LOOH)
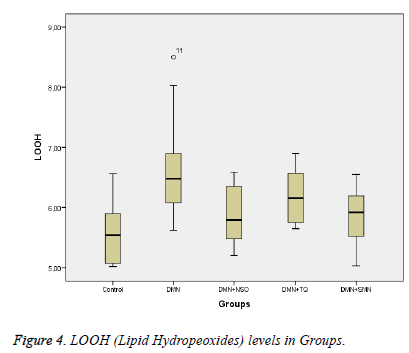
Lipid hydroperoxide (LOOH) levels were measured with the ferrous ion oxidation–xylenol orange method as previously described [17]. The results are expressed as μmol/g protein (Figure 4).
Assay of Total Thiol concentrations
The Total Thiol concentrations were measured by the methods originally described by Ellman and modified by Hu [18,19]. After measurement of total protein concentrations with Lowry method in renal tissues, the resulting unit of TAS was converted to μmol/g protein [20].
Statistical analysis
Analysis of data was made via IBM Statistical Package for Social Sciences version 20 (SPSS Inc., Chicago, IL, USA) and normality of distribution for the quantitative data was controlled using Kolmogorov-Smirnov test. Parametric tests were applied to data of normal distribution, while nonparametric tests were administered for data devoid of normal distribution. One-way ANOVA test was used for comparison of groups with independent continuous variables and Duncan test was used for comparison tests. Continuous data were expressed as mean ±standard deviation and differences associated with a p value <0.05 was considered as statistically significant.
Results
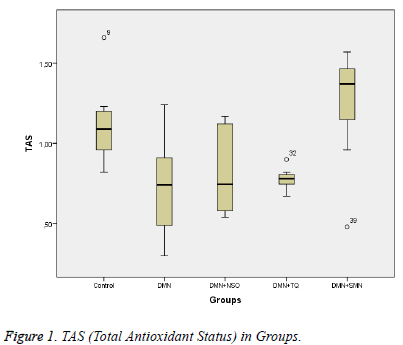
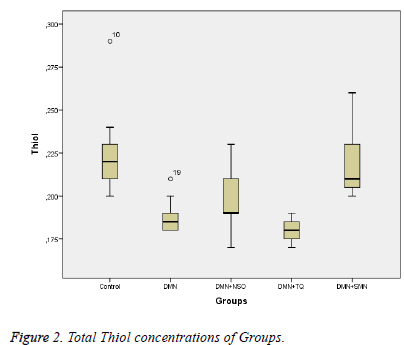
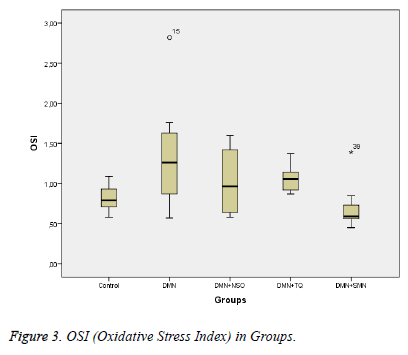
Data collected in this study was found to have a normal distribution (p values >0,05 for all parameters). Five homogeneous groups were constituted with respect to results of Duncan test (Table 1). Analysis of variance yielded that there was a remarkable difference between groups in terms of TAS, OSI, Total Thiol and LOOH (p values were <0,001; 0,014; <0,001and 0,003 respectively) and was no remarkable difference between groups in terms of TOS (p=0,575) (Table 1 and Figures 1-3).
| parameters | DMN | DMN+NSO | DMN+TQ | DMN+SMN | CONTROL | p values* |
|---|---|---|---|---|---|---|
| TAS (mmol Trolox equivalent/g protein) | 0,71±0,28 | 0,81±0,26 | 0,78±0,07 | 1,23±0,38 | 1,12±0,23 | <0,001 |
| TOS (µmol H2O2 equivalent/g protein) | 8,1±1,56 | 7,56±0,91 | 8,3±1,7 | 7,8±0,63 | 7,71±0,87 | 0.575 |
| OSI (arbitrary unit) | 1,35±0,65 | 1,04±0,39 | 1,06±0,16 | 0,72±0,32 | 0,81±0,17 | 0.014 |
| LOOH (µmol/g protein) | 6,7±0,92 | 5,87±0,5 | 6,19±0,48 | 5,85±0,52 | 5,57±0,51 | 0.003 |
| Total Thiol (mmol/g protein) | 0,19±0,01 | 0,19±0,02 | 0,18±0,01 | 0,22±0,02 | 0,23±0,03 | <0,001 |
| Abbreviations: LOOH, Lipid hydroperoxides; OSI, Oxidative stress index; TAS, Total antioxidant status; TOS, Total oxidant status; | ||||||
| *obtained by using One-Way ANOVA test | ||||||
Table 1: Biochemical Characteristics of Groups.
When DMN Group was compared to other groups;;
Between DMN Group and Control Group:While TAS and Total Thiol were lower (p values were 0,011 and <0,001 respectively), OSI and LOOH was higher in DMN group vs. in Control Group (p values were 0,035 and 0,002 respectively).
Between DMN Group and DMN+NSO Group: LOOH was significantly lower in DMN+NSO group (p=0,046). A remarkable difference was not found in other parameters (p>0.05 for all parameters) (Figure 4).
Between DMN Group and DMN+TQ Group: None of the parameters was found significantly different (p>0.05 for all parameters).
Between DMN Group and DMN+SMN Group: In DMN+SMN group, TAS and total thiol concentrations were significantly higher (p=0.002 and p=00.7, respectively), while OSI and LOOH was found significantly lower (p=0.022 and p=0.08, respectively).
Discussion
In this study, TAS and Total Thiol were lower and OSI and LOOH were higher in DMN group than in control group. Furthermore, TAS and Total Thiol were lower and OSI was higher in DMN group compared to DMN+SMN Group. To our results, we can say that DMN makes an injury in the kidney tissue and SMN reduces this damage. The balance between the oxidant and antioxidant defenses in the body has important health implications. Aerobic cells have developed their own defense system, the antioxidant system, which includes enzymatic (SOD, GPx) and nonenzymatic components, including albumin, Ceruloplasmin, Total Thiol groups and vitamin E to detoxify the endogenous and exogenous oxidative challenges during aerobic metabolism or when they encounter stress-inducing agents such as LOOH and MDA [21-23].
The components of oxidative and antioxidative system were not evaluated separately in our study. However, there are some studies that these components were evaluated separately. Satish et al. have reported that SOD, CAT and GPx activities were significantly lower in rats with hepatocellular damage was inducted with DMN [1]. It has reported that peroxide radicals are formed and thiol groups react with peroxide radicals in the first step of oxidative reactions and then the level of thiol groups decrease [24]. Boroushaki et al. have reported the lower levels of thiol groups in nephrotoxicity [25]. There was not a significant increase in TOS in the animals administered DMN alone. This can be explained with consumption of antioxidants to prevent an increase in TOS as well as in LOOH, which is a component of TOS. The decrease in the TAS and Total Thiol concentrations supports the results. In addition, OSI, which is calculated with TAS/TOS ratio was significantly increased. Like TAS, TOS includes many components such as hydroxyl (OH), hydrogen peroxide (H2O2), singlet oxygen (O2 ↑↓), lipid hydroperoxide (LOOH), and superoxide (O2- ) [15-18].
In the study of Sathish et al., it was reported that DMN raised the levels of LOOH. Beside TAS levels, TOS levels may increase with higher doses of DMN administration than our study. Koc et al. investigated the effects of statins on the rats in which the noise-induced oxidative stress and reported that the statins increased the activities of antioxidant enzymes (SOD and GPx) and decreased the levels of oxidative stress parameters but it was not significant. However, they pointed out that this result could be related to the statin dose and a significant decrease could occur with higher statin doses [26]. In a study that investigated the effects of simvastatin on the lipid peroxidation and antioxidant parameters in patients with peripheral arterial disease, it was reported that statins show synergistic action with antioxidants to decrease the oxidative stress by increasing antioxidant levels and decreasing LOOH and MDA levels significantly [24].
There are several studies about the protective effects of statins on nephrotoxicity. Maheshwari et al. reported that simvastatin has protective effect on the cisplatin-induced nephrotoxicity. They supported these results by the recovery of the decayed renal function tests and lipid peroxidation and the recovery of decreased antioxidant SOD and CAT activities related to toxicity, and by histopathological findings [9]. In a metaanalysis study, it was reported that high-dose statin decreased the incidence of contrast-induced acute kidney injury [27]. Similar results were reported in another study in which the protective effect of statins was found to have a relation with anti-inflammatory and anti-apoptotic properties [17]. In their study, Santos et al. have confirmed the renoprotection with antioxidant principle of the simvastatin in acute kidney injury induced by sepsis in an experimental model [28,29].
In our study, renal function tests and hystopathological findings were not evaluated. Therefore, severity of the nephrotoxicity was not determined. However, decreasing of TAS levels and increasing of OSI levels in DMN group, and increasing of TAS and decreasing of OSI levels with injection of SMN support the protective effect of SMN on renal damage. Our results are in correlation with Maheshwari et al. findings [9]. To our results, administration of DMN with NSO only prevented from an increase in LOOH, but did not prevent from the decrease of TAS and Thiol concentrations and the increase of TOS and OSI. Many studies showed that phenolic compounds have antioxidant and free radical scavenging properties. Nigella sativa is a plant that includes phenolic compounds as well [1]. It includes fixed oil and volatile oil. Nigella sativa oil has a complex structure that is composed of fatty acids, vitamins, pigments, and volatile components. Also Nigella sativa includes major active constituents such as thymoquinone, thymol and dithymoquinone that has antioxidant effects and free oxygen scavenging properties [30]. In the study of Abdel Daim et al. in which the effects of NSO on the oxytetracycline (from the group of tetracycline) induced hepatonephrotoxicity in rabbits was investigated, they reported an increase in the antioxidant enzymes such as GSH, SOD and CAT, and a decrease in lipid peroxidation markers such as lipid hydroperoxide and MDA. Furthermore, these results were explained by the antioxidant effects of NOS by inhibition of lipid peroxidation or by scavenging of free oxygen radicals or by increasing SOD and CAT enzyme activities indirectly [30].
Havakhah et al. showed in their study that investigated the effect of NOS on ischemia-reperfusion injury (IRI) in rats that MDA and DNA damage were decreased and the reduced thiol levels were increased [31]. Administrating DMN with TQ did not prevent from an increase in oxidative stress, also did not improve the antioxidant parameters. Thymoquinone (TQ; 2- isopropyl-5-methyl-1,4-benzoquinone), the active compound of nigella sativa, is shown by many studies to have antibacterial, antihypertensive, antidiabetic, neuroprotective, anti-inflammatory ve antiapoptotic properties [3]. In the study of Elsherbiny et al., it was shown that Doxorubucin (DOX), a chemotherapeutic agent, protected the renal tissue against DOX by capturing free radical with its quinone structure, by increasing the molecules which control the antioxidant molecules such as nuclear factor erythroid 2-related factor and by decreasing lipid peroxidation in renal tissue and increasing antioxidant enzyme activity in the rats with DOX-induced nephrotoxicity [32].
Ahmad et al. investigated the effects of compounds of Nigella Sativa, methanolic extract (ME) and volatile oil (VO), and their constituents such as thymoquinone (TQ) and limonene (LMN) on the oxidative stress in rats and found that methanolic extract (ME) was more effective than TQ [30].
When the protective effects of SMN, NSO and TQ on the DMN-induced kidney damage were compared, SMN showed the most protective effect with the doses used in this study. While NSO showed partial protective effect, TQ was not found protective. This can be due to the doses used or mechanisms of affect. Higher effectiveness of NSO than TQ may be related to the compounds included except TQ.
Conclusion
In conclusion, SMN has prevented the kidney damage via its antioxidant effect. Similar studies may been designed with different doses of DMN, NSO, TQ and SMN on this subject.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Sathish P, Paramasivan V, Palani V, Sivanesan K.N-acetylcysteine attenuates dimethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol 2011;654: 181-186.
- Bhattacharjee A, Lappi VR, Rutherford MS, Schook LB. Molecular dissection of dimethylnitrosamine (DMN)-induced hepatotoxicity by mRNA differential display. Toxicol Appl Pharmacol 1998;150:186-195.
- Aydin MS, Kocarslan A, Kocarslan S, Kucuk A, Eser I, Sezen H, Buyukfirat E, Hazar A. Thymoquinone protects end organs from abdominal aorta ischemia/reperfusion injury in a rat model. Rev Bras Cir Cardiovasc 2015;30:77-83.
- Bayrak O, Bavbek N, Karatas OF, Bayrak R, Catal F, Cimentepe E, Akbas A, Yildirim E, Unal D, Akcay A. Nigella sativa protectsagainst ischaemia/reperfusion injury in rat kidneys. Nephrol Dial Transplant 2008;23:2206-2212
- Blanco-Colio LM, Tuñón J, Martín-Ventura JL, Egido J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int 2003;63:12-23.
- Gandhi S, Mosleh W, Abdel-Qadir H, Farkouh ME. Statins and contrast-induced acute kidney injury with coronary angiography. Am J Med 2014;127:987-1000.
- Chyou AC, Thodge A, Feldman DN, Swaminathan RV. Statins in the prevention of contrast-induced nephropathy. Curr Treat Options Cardiovasc Med 2015;17:375.
- Maheshwari RA, Balaraman R, Sailor GU, Sen DB. Protective effect of simvastatin and rosuvastatin on trinitrobenzene sulfonic acid-induced colitis in rats. Indian J Pharmacol 2015;47:17-21.
- Maheshwari RA, Sailor GU, Patel L, Balaraman R. Amelioration of cisplatin-induced nephrotoxicity by statins.Indian J Pharmacol 2013;45:354-358.
- Zinellu A, Sotgia S, Mangoni AA, Sanna M, Satta AE, Carru C. Impact of cholesterol lowering treatment on plasma kynurenine and tryptophan concentrations in chronic kidney disease: relationship with oxidative stress improvement. Nutr Metab Cardiovasc Dis 2015;25:153-159.
- Alupei MC, Licarete E, Patras L, Banciu M. Liposomal simvastatin inhibits tumor growth via targeting tumor-associated macrophages-mediated oxidative stress. Cancer Lett 2015;356:946-952.
- Usui H, Shikata K, Matsuda M, Okada S, Ogawa D, Yamashita T, Hida K, Satoh M, Wada J, Makino H. HMG-CoA reductase inhibitor ameliorates diabetic nephropathy by its pleiotropic effects in rats. Nephrol Dial Transplant. 2003;18:265-272.
- Harris KP, Purkerson ML, Yates J, Klahr S. Lovastatin ameliorates the development of glomerulosclerosis and uremia in experimental nephrotic syndrome. Am J Kidney Dis 1990;15:16-23.
- Li C, Yang CW, Park JH, Lim SW, Sun BK, Jung JY, Kim SB, Kim YS, Kim J, Bang BK. Pravastatin treatment attenuates interstitial inflammation and fibrosis in a rat model of chronic cyclosporine-induced nephropathy. Am J Physiol Renal Physiol 2004;286:46-57.
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem 2005;38:1103–1111.
- Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 2004; 37: 112-119.
- Nourooz-Zadeh J. Ferrous ion oxidation in presence of xylenol orange for detection of lipid hydroperoxides in plasma. Methods Enzymol 1999;300:58-62.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70-77.
- Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994;233:380-385.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-275.
- Cikman O, Ozkan A, Aras AB, Soylemez O, Alkis H, Taysi S, Karaayvaz M. Radioprotective effects of Nigella Sativa oil against oxidative stress in liver tissue of rats exposed to total head irradiation. J Invest Surg 2014; 27: 262-266.
- Hu C, Sun L, Han Y, Fu X, Xiong X, Xu X, Liu Y, Yang S, Liu F, Kanwar YS. Insights into The Mechanisms Involved In The Expression And Regulation of Extracellular Matrix Proteins In Diabetic Nephropathy. Curr Med Chem. 2015;22:2858-2870.
- Buyukokuroglu ME, Taysi S, Polat F, Gocer F. Mechanism of the beneficial effects of dantrolene sodium on ethanolinduced acute gastric mucosal injury in rats. Pharmacol Res 2002;45: 421-425.
- Strzy?ewski KW, Pioru?ska-Stolzmann M, Majewski W, Kasprzak M, Strzy?ewski W. Effect of surgical treatment on lipid peroxidation parameters and antioxidant status in the serum of patients with peripheral arterial disease. Dis Markers 2013;35:647-652.
- Boroushaki MT, Mollazadeh H, Rajabian A, Dolati K, Hoseini A, Paseban M, Farzadnia M. Protective effect of pomegranate seed oil against mercuric chloride-induced nephrotoxicity in rat. Ren Fail 2014;36:1581-1586.
- Koc ER, Ersoy A, Ilhan A, Erken HA, Sah?n S. Is rosuvastatin protective against on noise-induced oxidative stress in rat serum? Noise Health 2015;17:11-16.
- Ukaigwe A, Karmacharya P, Mahmood M, Pathak R, Aryal MR, Jalota L, Donato AA. Meta-analysis on efficacy of statins for prevention of contrast-induced acute kidney injury in patients undergoing coronary angiography. Am J Cardiol 2014; 114:1295-1302.
- Santos Fdo N, Watanabe M, Vasco CF, Fonseca CD, Vattimo Mde F. [Antioxidant protection of statins in acute kidney injury induced by sepsis]. Rev Esc Enferm USP 2014;48:820-826.
- Ahmad S, Beg ZH. Mitigating role of thymoquinone rich fractions from Nigella sativa oil and its constituents, thymoquinone and limonene on lipidemic-oxidative injury in rats. Springerplus. 2014;25:316.
- Abdel-Daim MM, Ghazy EW. Effects of Nigella sativa oil and ascorbic acid against oxytetracycline-induced hepato-renal toxicity in rabbits. Iran J Basic Med Sci 2015;18:221-227.
- Havakhah S, Sadeghnia HR, Hajzadeh MA, Roshan NM, Shafiee S, Hosseinzadeh H, Mohareri N, Rad AK. Effect of Nigella sativa on ischemia-reperfusion induced rat kidney damage. Iran J Basic Med Sci2014;17:986-992.
- Elsherbiny N, El-Sherbiny M. Thymoquinone attenuates Doxorubicin-induced nephrotoxicity in rats: Role of Nrf2 and NOX4. Chem Biol Interact 2014;28: 102-108.