ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2016) Volume 27, Issue 2
Quantitative determination of Radix ophiopogonison and its protective effect on intestinal ischemia reperfusion-induced lung injury in rats.
| Wen-jie Fan1, Xiao-hui Sun2, Yong Sun3*, Xian-min Jiang3 1Department of Anesthesia, Qingdao Women and Children’ Hospital, 217#, Liaoyang Xi Road, Qingdao 266000, PR. China 2Department of Hepatopathy,Qingdao Infectious Disease Hospital, 9#, Fushun Road, Qingdao 266000, PR. China 3Department of Pediatric Surgery, Qingdao Women and Children’ Hospital, 217#, Liaoyang Xi Road, Qingdao 266000, PR. China |
| Corresponding Author: Yong Sun, Department of Pediatric Surgery, Qingdao Women and Children’ Hospital, PR. China |
| Accepted: February 11, 2015 |
To establish a RP-HPLC method for the determination of ophiopogonin A content in Radix ophiopogonison; and to observe the intervention effect of Radix ophiopogonison extract on acute lung injury after intestinal ischemia reperfusion. Synergi 4 μ Hydro-RP 80A column (250 mm × 4.6 mm, 5 μm) is used, column temperature is 25°C, mobile phase is water-acetonitrile (volume ratio 62:38), flow rate is 1.0 mL/min and detection wavelength 208 nm. Rat model of intestinal ischemia reperfusion is established, and then the rats are randomized into control group, ischemia reperfusion group and Radix ophiopogonison intervention group. Total protein content in Bronchoalveolar Lavage Fluid (BALF), dry/wet weight ratio (D/W) of lung tissues, peroxidase (MPO) content in lung tissues and Malondialdehyde (MDA) content in plasma are measured. Mass concentration of ophiopogonin A shows a good linear relationship (r=0.9999) with the peak area at a range of 0.020~0.200 mg/mL, and the average recovery is 99.75% (RSD=0.22%, n=9). Compared with the ischemic reperfusion group, BALF total protein content increases significantly, while lung D/W decreases significantly in the intervention group. The content determination method established herein is simple and accurate, which can be used for the quality control of Radix ophiopogonison medicinal material. Radix ophiopogonison extract can significantly reduce the degree of acute lung injury.
Keywords |
||||||||
| Radix ophiopogonison, Ophiopogonin A, Ischemia reperfusion, Intestinal ischemia reperfusion, Lung protection. | ||||||||
Introduction |
||||||||
| Radix ophiopogonison is the dried root tuber of Ophiopogon japonicus (Thunb.) Ker-Gawl in the genus Ophiopogon of the family Liliaceae, which is a common Yin-nourishing traditional Chinese medicine; originally recorded in the "Shen Nong's Herbal Classic", it has Yin-nourishing, fluid-engendering, lung-moistening and heart-clearing functions, and can be used for lung dryness, dry cough, Yin deficiency, tuberculosis, throat impediment, sore throat, fluid deficiency, thirst, vexation, insomnia and other symptoms [1]. Modern studies have found that Radix ophiopogonison mainly contains polysaccharides, steroidal saponins and homoisoflavonoids [2-5], and has anti-cancer, anti-diabetic and fatty acid synthesis inhibitory effects [3-5]. Peroxidase (MPO) and plasma lipid peroxidation product Malondialdehyde (MDA) play important roles in the pathogenesis of Multiple Organ Dysfunction Syndrome (MODS) caused by intestinal ischemia reperfusion-induced remote organ injury [6,7]. | ||||||||
| In this experiment, HPLC method is used to determine the ophiopogonin A content in Radix ophiopogonison, thus providing a new approach for the quality control of Radix ophiopogonison medicinal material. Meanwhile, a rat model of intestinal ischemia reperfusion is used to observe the effects of Radix ophiopogonison extract on lung tissue MPO and plasma MDA contents in rats, in order to explore the pathogenetic mechanism of lung injury and the protective effect of Radix ophiopogonison extract | ||||||||
Material and Methods |
||||||||
| Instruments | ||||||||
| Agilent 1200 HPLC system; EYELA N1000 rotary evaporator; Sigma 3K15 centrifuge™, Germany; HH-4 electric heated thermostatic water bath (Guohua Scientific Instrument Factory, Changzhou); METTLER TOLEDO electronic analytical balance. | ||||||||
| Herb and drug | ||||||||
| Radix ophiopogonison was purchased from Qingping Pharmaceutical Company in Guangzhou, which was identified by Professor Wang Weizhi at Guangzhou University of Chinese Medicine as the dried root tuber of Ophiopogon japonicus (Thunb.) Ker-Gawl. The herb was dried in a 60°C oven for 6 h, then pulverized, passed through a 40-mesh sieve, and stored in a desiccator for later use. Ophiopogonin A reference substance, with a purity of 99.5%, was purchased from the National Institute for the Control of Pharmaceutical and Biological Products. MDA and MPO kits were purchased from Meijin Biotechnology Co., Ltd., Tianjin China. | ||||||||
| Reagents | ||||||||
| Acetonitrile and methanol were of HPLC grade (Kaimei Technology Co., Ltd., Jiangsu); water used for HPLC was purified water (self-prepared); and other reagents were all of analytical grade (Nanjing Chemical Reagent Factory). | ||||||||
| Preparation of animal model | ||||||||
| Intestinal ischemia reperfusion injury model was replicated by referring to Moore et al.'s method [6]. Healthy male SD rats were fed routinely, and fasted with water for 12 h before the experiment. The weight of the rat is 235~270 g, Average is 250 g. After anesthetization by intraperitoneal injection of 100 mg/kg ketamine hydrochloride, right carotid artery cannulation was performed under sterile conditions for continuous monitoring of arterial blood pressure and ECG. Abdomen was incised along the midline; next, Superior Mesenteric Artery (SMA) was isolated, and occluded with noninvasive vascular clamp; then, incision was sutured. 45 min later, the artery clamp was removed through the original incision site to restore blood supply. All experimental procedures were approved by the Animal Research Ethics Committee. | ||||||||
| Animal grouping | ||||||||
| Animals were randomly divided into three groups: | ||||||||
| • Ischemia reperfusion group (n=16) | ||||||||
| • Control group (n=16): surgical procedures were the same as the ischemia reperfusion group except that SMA was not occluded | ||||||||
| • Radix ophiopogonison intervention group (n=16): Radix ophiopogonison extract was prepared into a 600 mg/kg suspension, and administered through gastric tube 30 min before restoration of blood supply | ||||||||
| Chromatographic conditions | ||||||||
| Column: Synergi 4 μ Hydro-RP 80A (250 mm × 4.6 mm, 5 μm); column temperature: 25°C; mobile phase: water-acetonitrile (volume ratio 62:38); flow rate: 1.0 mL/min; detection wavelength: 208 nm; injection volume: 10 μL. | ||||||||
| System suitability test | ||||||||
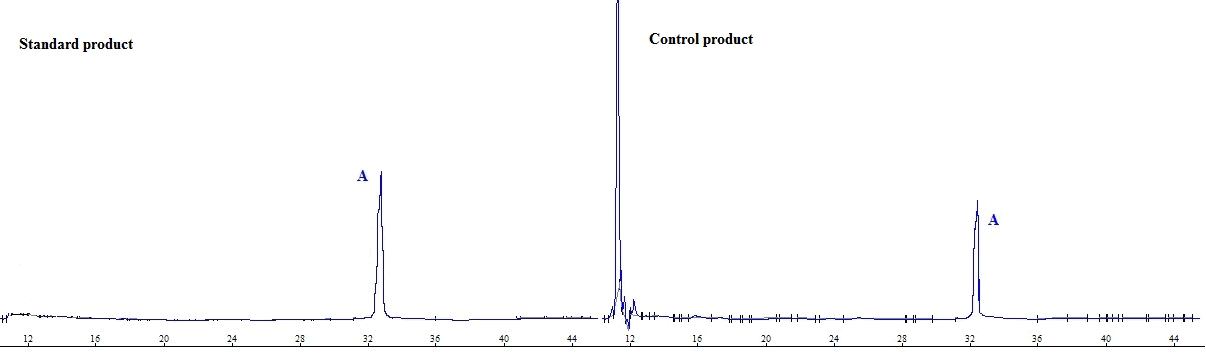
| Under the chromatographic conditions of this experiment, retention time of ophiopogonin A was about 32.12 min, symmetry factor was 0.92, number of theoretical plates was calculated based on ophiopogonin A to be not less than 4,000, and resolution of adjacent peaks was greater than 1.5. Typical chromatogram is shown in figure 1. | ||||||||
| Plotting of standard curve | ||||||||
| About 9.0 mg of ophiopogonin A reference substance was accurately weighed, placed in a 25 ml volumetric flask, dissolved in methanol, diluted to the mark, and shaken well as the stock solution. Reference stock solution was diluted in different proportions to give 0.020, 0.060, 0.100, 0.160 and 0.200 mg/ml solutions, and injected for analysis. Standard curve was plotted with peak area (Y) versus concentration (X) of ophiopogonin A, and its regression equation was Y=2.4567 × 106-4.145 × 103, r=0.9999. Ophiopogonin A had a good linearity between 0.020~0.200 mg. | ||||||||
| Preparation of sample solution | ||||||||
| 2.0 g of Radix ophiopogonison medicinal material was accurately weighed, placed in a round bottom flask, added with 24 mL of 75% ethanol, and refluxed in a water bath for 2 h, then filtered; the extraction was repeated three times. Filtrates were combined, and evaporated to dryness under reduced pressure. The residue was washed with 10 mL of water, quantitatively transferred to a separating funnel, and extracted three times with 20 mL of N-butanol. N-butanol layers were combined, and evaporated to dryness under reduced pressure. The residue was then dissolved in HPLC grade methanol, quantitatively transferred to a 10 mL volumetric flask, diluted to the mark, and shaken well. The solution was centrifuged at 10,000 rpm for 5 min, and filtered through a 0.45 μm membrane. 2 mL of the filtrate was placed into a 5 mL volumetric flask, diluted to the mark with HPLC grade methanol, and shaken well to give the sample solution. | ||||||||
| Precision test | ||||||||
| Precision of instrument | ||||||||
| Reference solution was successively injected six times under the above chromatographic conditions, and the RSD of ophiopogonin A peak area was calculated to be 1.6% (n=6). | ||||||||
| Reproducibility of method | ||||||||
| Six samples were prepared in parallel as per the method under "Preparation of sample solution", ophiopogonin A content was determined, and RSD was calculated to be 1.6% (n=6). | ||||||||
| Sample solution stability test | ||||||||
| Test solution was taken, placed at room temperature, and then injected at 0, 2, 4, 8, 12 and 24 h, respectively, for analysis. RSD of ophiopogonin A peak area was 1.3% (n=6), indicating that the sample solution was relatively stable within 24 h. | ||||||||
| Recovery test | ||||||||
| Appropriate amount of known content of test solution was taken, added precisely with an appropriate amount of reference stock solution, and determined as per the method. Average recovery was 99.75%, and RSD=0.22% (n=9). The results are shown in table 1. | ||||||||
| Content determination | ||||||||
| Sample solution was prepared as per the method under "Preparation of sample solution", and ophiopogonin A content was determined by two-point external standard method. The results are shown in table 2. | ||||||||
| Indices and methods for measurement of lung protective effect | ||||||||
| Total protein in BALF was measured by dry chemistry method; plasma MDA content was measured as per MDA kit instructions; and MPO content in lung tissues was measured by colorimetry using H2O2 reaction product [8,9]. | ||||||||
| Statistical method | ||||||||
| All measurement data were expressed as x? ± s; non-parametric rank sum test and ANOVA were performed using STATA 4.0 statistical software; P <0.05 was considered statistically significant. | ||||||||
Results |
||||||||
| Changes in BALF total protein content and lung D/W ratio of rats in different groups: rats in the control group had the highest content of total protein in BALF; BALF total protein content for the intervention group was higher than the ischemia reperfusion group, and the difference was statistically significant. Lung D/W value was the highest in the control group; intervention group had a lung D/W value higher than the ischemia reperfusion group, and the difference was statistically significant (Table 3). | ||||||||
| Changes in lung tissue MPO and plasma MDA levels of rats in different groups: Lung tissue MPO and plasma MDA levels increased rather markedly in the ischemia reperfusion group compared with the control group. Administration of Radix ophiopogonison before ischemia could significantly lower both lung tissue MPO and plasma MDA levels (Table 4). | ||||||||
Discussion |
||||||||
| Ophiopogonin A reference solution with an appropriate concentration was prepared with acetonitrile, and UV scanned at 200 nm~400 nm; maximum absorption appeared at 208 nm. In order to improve detection sensitivity, 208 nm was selected as the detection wavelength. Column temperature, as well as composition, proportion and flow rate of mobile phase were adjusted according to the literatures [10-12]; the results showed that when mobile phase was water-acetonitrile (62:38, v/v) and flow rate was 1.0 ml/min, column temperature was 25°C, ophiopogonin A separated well from other substances, and analysis time was moderate. There have been rather many reports on ophiopogonin D among studies on Radix ophiopogonison. This study finds that ophiopogonin A has a protective effect on lung injury. In order to clarify the activity of ophiopogonin A and to control its quality, this paper reports a method for determination of ophiopogonin A content. | ||||||||
| Generally speaking, acute lung injury caused by extrapulmonary factors is a manifestation of Systemic Inflammatory Response Syndrome (SIRS), which is an inflammatory response of lung tissues caused by production of inflammatory cytokines and biological agents upon activation of neutrophils, etc. in the lung capillaries by interaction of multiple activity mediators [13]. Neutrophils are the major inflammatory cells; after such cells are activated, there will be sudden increase in oxidative metabolism, giving rise to the production of excessive reactive oxygen species including superoxide anion (O2-), hydrogen peroxide (H2O2), hydroxyl radical (HO•) and MPO.H2O2 can be oxidized to form proteins, phospholipids, metabolic enzyme DNA and intercellular matrix components (collagen and hyaluronic acid) of cellular components, leading to platelet and vascular dysfunctions. After polyunsaturated fatty acids in the macromolecular biofilm phospholipids are attacked, lipid peroxidation will occur, forming certain toxic lipid peroxidation products including MDA, thereby further destroying the structure and function of cells. | ||||||||
| Intestinal ischemia reperfusion is a common pathophysiological process in anorectal surgery, not only causing damage to the intestine itself, but also leading to failure of multiple organs, among which intestine is the earliest involved organ. Therefore, the search for drugs with a protective action on intestinal ischemia reperfusion injury is particularly important. In our experiment, we used a rat model of intestinal ischemia reperfusion, took BALF protein content and lung D/W ratio as the important indices reflecting changes in pulmonary capillary permeability, and observed changes in plasma levels of MDA, which allowed understanding of the intensity of oxidation reaction in organisms to some extent. MPO mainly exists in the neutrophil lysosomes, which is an enzyme necessary for scavenging of oxygen free radicals; elevated levels of MPO in lung tissues suggests increased neutrophil accumulation in lung tissues and production of large amounts of oxygen free radicals in lung tissues [14,15]. | ||||||||
Conclusion |
||||||||
| We found from the experimental results that with the incidence of intestinal ischemia reperfusion, indices like BALF protein content, lung tissue MPO content, plasma MDA content and lung tissue D/W ratio tended to be normal in the Radix ophiopogonison intervention group. This indicates that the imbalance of oxidation-antioxidation system is a major pathophysiological process of acute lung injury after intestinal ischemia reperfusion; and Radix ophiopogonison significantly reduces the severity of acute lung injury by inhibiting neutrophilic exudation in lung tissues and by its own antioxidant effect. | ||||||||
Tables at a glance |
||||||||
|
||||||||
Figures at a glance |
||||||||
|
||||||||
References |
||||||||
|