ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 14
Research on the inhibitory effect of metformin on human oral squamous cell carcinoma SCC-4 and CAL-27 cells and the relevant molecular mechanism
Yiming Chen#, Liu Liao#, Jingjing Qiu, Yongze Zheng, Shunli Tang, Rongrong Li, Dan Xie, Xiaoqiu Zou, Shuzhen Zheng, Xiang tao Weng* and Wenjing Zhang*
Guangzhou University of Chinese Medicine, Guangzhou, PR China
#These authors contributed equally to this work
- *Corresponding Author:
- Xiang tao Weng
Guangzhou University of Chinese Medicine, PR China
Wenjing Zhang
Guangzhou University of Chinese Medicine, PR China
Accepted date: June 19, 2017
Objective: To study the metformin in human oral squamous cell carcinomas SCC-4 and CAL-27 cell inhibitory effect and its molecular mechanism.
Methods: With human oral squamous cell carcinomas Squamous Cell Carcinoma-4 (SCC-4) and CAL-27 subcutaneous transplantation tumor cells and nude mouse models as the research subjects, we carried out MTT assay, flow cytometry assay and Western blot assay methods, so as to investigate the effects of metformin on cell proliferation, activity, clone formation and cycle in in-vitro culture of human oral squamous cell carcinomas SCC-4 and CAL-27 cells, and to explore its molecular mechanism.
Results: The activity of oral squamous cell carcinomas SCC-4 and CAL-27 cells in different concentration intervention after 48 h activity were reduced (p<0.05) in comparison with the control group, and presented an obvious concentration-dependent manner. By treatment of metformin in different concentration for two weeks, compared with the normal control group, oral squamous cell carcinomas SCC-4 and CAL-27 cell clone number were significantly reduced (p<0.05), and the reduction was in an obvious concentration-dependent manner.
Conclusion: Metformin can reduce the activity of oral squamous cell carcinomas SCC-4 and CAL-27 cell, inhibit the proliferation and clone formation of oral squamous cancer cell, and the oral squamous cell carcinomas SCC-4 and CAL-27 cell growth is stopped in G0/G1 phase, thereby activating AMPK signaling pathways and inhibiting mTOR signaling pathways.
Keywords
Metformin, Oral squamous cell carcinomas, SCC-4 cells, CAL-27 cells, Molecular mechanisms
Introduction
Metformin is a kind of insulin sensitizer that has been widely applied in the treatment of type ii diabetes mellitus. However, recent studies have found significant inhibitory or blocking effect of metformin on the capability of proliferation, cell activity, clone formation and cell cycle of tumor cells, such as prostatic cancer [1], breast cancer [2] and gastric cancer [3]. Nevertheless, there remain few studies reporting the inhibitory effect of metformin on human oral squamous cell carcinoma cells. In this study, we selected the human oral squamous cell carcinoma SCC-4 and CAL-27 cells as the subjects to observe the effect of metformin on the in-vitro proliferation capability, cell activity, clone formation and cell cycle of human oral squamous cell carcinoma SCC-4 and CAL-27 cells, and attempted to discover the potential molecular mechanism, aiming to provide the evidence for following in-vivo anti-tumor studies.
Material and Methods
Experiment material
Cell strain: Oral squamous cell carcinoma SCC-4 cell strain and CAL-27 cell strain that were purchased from the Cell Bank, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China.
Experiment animals: 25 BALB/C-nu male nude mice were purchased from Beijing HFK Bioscience Co., Ltd. The age of mice for experiment was not younger than 4 weeks, and the average age was (4.51 ± 0.54 w). The weight of mice ranged from 18 to 25 g, and the average weight was (21.47 ± 2.87 g). Experiment drugs: Metformin that was purchased from Sigma- Aldrich Co., Ltd, USA. Major apparatus: CO2 cell incubator, clean bench, ultra-pure water purifier, electronic analytical balance, centrifuge, electrophoresis apparatus, electronic transfer box, etc. Major experiment reagents: RPMI-1640 (Roswell Park Memorial Institute) culture media, fetal calf serum, trypsin, MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide), paraformaldehyde, crystal violet, etc.
Experiment methods
Various experimental solutions were prepared for the resuscitation, culture and passage of oral squamous cell carcinoma SCC-4 cell strain and CAL-27 cell strain, including the RPMI-1640 culture medium, Dulbecco's Modified Eagle Medium (DMEM), Phosphate Buffer Saline (PBS), 0.25% trypsin, and 0.02% Ethylene Diamine Tetraacetic Acid (EDTA). Then we applied the MTT, cytometry, and Westernblot methods to investigate the effects of metformin on in-vitro proliferation capability, cell activity, clone formation and cell cycle of human oral squamous cell carcinoma SCC-4 cell and CAL-27 cell and clarify the relevant molecular mechanism.
(1) Detecting the in-vitro proliferation capability of cells via MTT method: Human oral squamous cell carcinoma SCC-4 cell and CAL-27 cell in the logarithmic phase were taken for regular digestion, centrifugation and resuspension to prepare the single cell suspension. Cells were inoculated onto the 96- well plate in 5 × 103/well. Plate was then transferred into an incubator for over 24 h of cell culture at 37°C in 5% CO2. Then the original medium in each well was removed and media with 2, 5, 10 and 20 mM metformin were added. After 2 w of regular culture, 20 μL MTT (5 mg/ml) was added onto each well followed by over 4 h of regular culture. The supernatant was discarded, and the Optical Density (PD) at 490 nm wavelength of each well was detected. The survival rate of cell was calculated using the following formula: survival rate=OD value of experiment group/OD value of control group ×100%.
(2) Detecting the clone formation of cells: Human oral squamous cell carcinoma SCC-4 cell and CAL-27 cell in the logarithmic phase were taken for regular digestion, centrifugation and resuspension to prepare the single cell suspension. Cells were inoculated onto the 6-well plate in 1 × 103/well. Plate was then transferred into an incubator for over 24 h of cell culture at 37°C in 5% CO2. Then the original medium in each well was removed, media containing 5% FBS and media with 2, 5, and 10 mM metformin were added. After 2 w of regular culture, the original media were removed and the plate was washed for 3 times using PBS, fixated using 4% paraformaldehyde, and then washed by PBS for 3 times After 5 min of staining using 0.1% crystal violet, the plate was carefully rinsed using deionized water until the solution turned into colorless and clear. Finally, we applied the high power digital camera to record the results, and analysis of clone formation of cells was carried out using Quantity One software. The rate of cell clone formation was calculated using the following formula: Formation rate of cell clone=Quantity of clone in experiment group/quantity of clone in control group × 100%.
(3) Detecting the cell cycle via flow cytometry: Human oral squamous cell carcinoma SCC-4 cell and CAL-27 cell in the logarithmic phase were taken for regular digestion, centrifugation and resuspension to prepare the single cell suspension. Cells were inoculated onto the 6-well plate in 2 × 105/well. Plate was then transferred into an incubator for over 24 h of cell culture at 37°C in 5% CO2. Then the original medium in each well was removed and media containing 5 mM metformin were added. After 12, 24 and 48 h of regular culture, cells were digested using trypsin followed by the centrifugation and collection of cells. Then cells were washed using PBS, and the ratio of cells in each cycle was analysed using flow cytometer and Modifit software.
(4) Detecting the relevant proteins in cells via Westernblotting method: Total protein of cell was extracted in accordance with the standardized method, and quantification of protein was also performed using Bradford colorimetry. After electrophoresis and membrane-transfer procedures, NC membrane was soaked in the blocking solution and then slowly shaken at room temperature for 1 h of blocking. Molecular weight of the protein was calculated according to the positions of protein marker. Then the first antibody specific to the target protein was added onto the membrane and the NC membrane was then shaken for incubation at 4°C overnight. Thereafter, goat anti-rabbit or goat anti-mouse secondary antibody was added onto the membrane according to the species origin for 1 h of incubation. ECL developer was prepared. After the tribuffer saline+Tween 20 (TBST) on the NC membrane was removed, 5 ml ECL developer was added onto the membrane followed by blow, beat and well-mixing. Then the ECL developer on the membrane was removed, and the membrane was then placed into a dark room equipped with Bio-Rad apparatus. The time of exposure was adjusted according to the brightness of stripes, and the pictures were collected using Image Lab software.
Statistical methods
Statistical analysis of data was then carried out using the SPSS 19.0 software. Measurement data were presented as mean ± standard deviation. Chi-square test was performed in comparison of count data. In this study, p<0.05 suggested that the difference had statistical significance.
Results
Inhibitory effect of metformin on the in-vitro proliferation capability of oral squamous cell carcinoma SCC-4 cell and CAL-27 cell
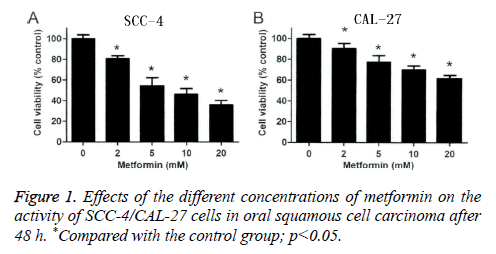
In this study, MTT detection was applied to detect the effects of metformin on oral squamous cell carcinoma SCC-4 cell and CAL-27 cell. According to Figure 1, we found that at 48 h after the intervention of metformin in different concentrations on oral squamous cell carcinoma SCC-4 cells, cell activities, when compared with the control group (p<0.05), were reduced by (19.51 ± 2.93%), (44.48 ± 7.85%), (54.83 ± 5.63%) and (63.94 ± 4.46%), respectively (Figure 1A); at 48 h after the intervention of metformin in different concentrations on oral squamous cell carcinoma CAL-27 cells, cell activities, when compared with the control group (p<0.05), were reduced by (10.48 ± 3.08%), (20.74 ± 2.73%), (38.25 ± 4.65%) and (47.49 ± 6.08%), respectively (Figure 1B). This indicated that metformin can significantly reduce the activity of oral squamous cell carcinoma SCC-4 cell and CAL-27 cell, inhibit the cell proliferation in oral squamous cell carcinoma, and the inhibitory pattern shows an obvious reliance on the concentration of metformin.
Blocking effect of metformin on the cell cycle of oral squamous cell carcinoma SCC-4 cell and CAL-27 cell
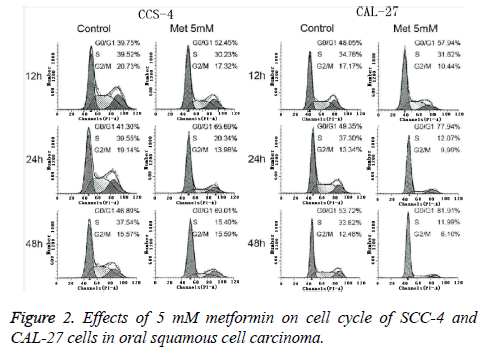
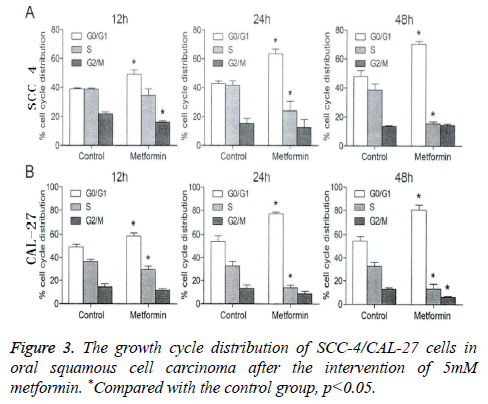
In this study, flow cytometry was performed for analyzing the blocking effect of metformin on the cell cycle of oral squamous cell carcinoma SCC-4 cell and CAL-27 cell. As shown in Figures 2 and 3, at 12 h, 24 h and 48 h after the intervention of 5 mM metformin for oral squamous cell carcinoma SCC-4 cell and CAL-27 cell, gradual increases were identified in the ratio of cells in G0/G1 phase over time, significantly higher than those in the control group at the same time point (p<0.05), but a gradual decrease was also seen in the ratio of cells in S and G2/M phases. This indicated that metformin can block the growth of oral squamous cell carcinoma SCC-4 cell and CAL-27 cell in G0/G1 phase.
Inhibitory effect of metformin on the clone formation of oral squamous cell carcinoma SCC-4 cell and CAL-7 cell
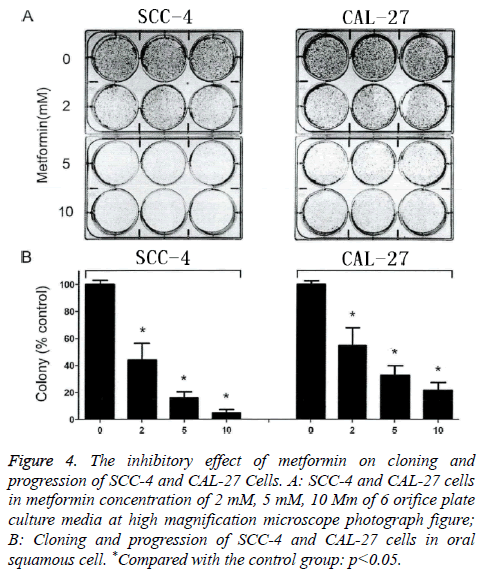
Figure 4A is the high-power microscopic images of cells in 6- well plate that were treated using 2 Mm, 5mM and 10 mM of metformin. From the Figure 4A, after 2 w of intervention using metformin in different concentrations, the quantity of clones was significantly decreased when compared with those in the control group. Even if the concentration of metformin was only 2 mM, the count of clone formations of the oral squamous cell carcinoma SCC-4 cell and CAL-27 cell was also significantly decreased by (54.83 ± 10.46%) and (42.42 ± 11.28%) in comparison with that in the control group, and the difference had statistical significance (p<0.05). Additionally, with an increase in the concentration of metformin, a gradual decrease was found in the count of clone formation of oral squamous cell carcinoma SCC-4 cell and CAL-27 cell in an obvious concentration-dependent manner (Figure 4B). All these results showed that metformin can significantly suppress the clone formation of oral squamous cell carcinoma SCC-4 cell and CAL-27 cell.
Figure 4: The inhibitory effect of metformin on cloning and progression of SCC-4 and CAL-27 Cells. A: SCC-4 and CAL-27 cells in metformin concentration of 2 mM, 5 mM, 10 Mm of 6 orifice plate culture media at high magnification microscope photograph figure; B: Cloning and progression of SCC-4 and CAL-27 cells in oral squamous cell. *Compared with the control group: p<0.05.
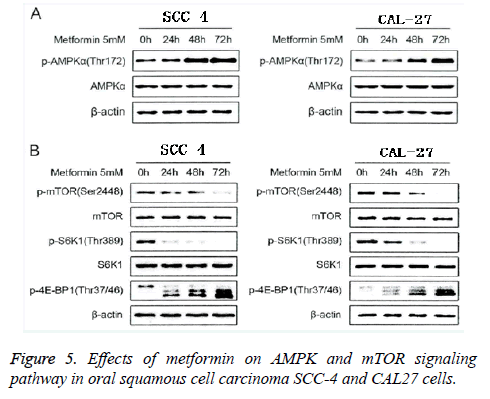
Effect of metformin on the AMPK/mTOR signal pathway of oral squamous cell carcinoma SCC-4 cell and CAL-27 cell
In this study, we extracted the total protein of oral squamous cell carcinoma SCC-4 cell and CAL-27 cell that were intervened using metformin in concentration of 5 mM in different time points (0 h, 24 h, 48 h and 72 h). With β-actin as the reference protein, we applied the Western-blot method to detect the expressions of AMPK/mTOR signal pathway, and the results were shown in Figure 5. From the Figure 5A, we found that after the intervention of metformin in concentration of 5 mM on the oral squamous cell carcinoma SCC-4 cell and CAL-27 cell, significant changes were observed in the total quantity of AMPKa, and significant increases were also identified in phosphorylation level of AMPKa (Thrl72) in a significant time-dependent manner. This indicated that metformin can activate the AMPK signal pathway in oral squamous cell carcinoma SCC-4 cell and CAL-27 cell. From Figure 5B, we could see that after the intervention of metformin in concentration of 5 mM on the oral squamous cell carcinoma SCC-4 cell and CAL-27 cell, obvious decreases were observed in the expressions of mTOR and the downstream target molecules, such as p-mTOR (Ser2448), p- S6Kl (Thr389) and p-4E-BPl (Thr37/46) in a significant timedependent manner. This suggested that metformin can inhibit the mTOR signal pathway in oral squamous cell carcinoma SCC-4 cell and CAL-27 cell. These results showed that metformin can inhibit the proliferation and growth of oral squamous cell carcinoma SCC-4 cell and CAL-27 cell through activating the AMPK signal pathway.
Discussion
Metformin is a kind of antidiabetic agent that has been widely applied in clinical practice, and is characterized by various advantages, such as the rapid decrease in the blood glucose, exact efficacy and few side reactions. Additionally, the research showed that metformin has a significant inhibitory effect on multiple tumor cells [4]. In this study, oral squamous cell carcinoma SCC-4 cell and CAL-27 cell were selected as the subjects, and the study showed that: At 48 h after the intervention of metformin in different concentrations on oral squamous cell carcinoma SCC-4 cells, cell activities, when compared with the control group (p<0.05), were reduced in an obvious concentration-dependent manner; after 2 w of intervention using metformin in different concentrations, the quantity of clones was significantly decreased when compared with those in the control group (p<0.05) in an obvious concentration-dependent manner; at 12 h, 24 h and 48 h after the intervention of 5 mM metformin for oral squamous cell carcinoma SCC-4 cell and CAL-27 cell, gradual increases were identified in the ratio of cells in G0/G1 phase over time in a time-dependent manner. These results are coincident with the research results of the inhibitory effect of metformin on other tumor cells [5-7].
Cell proliferation in tumor depends on the smooth implementation of cell cycles. Generally, cell cycles in tumor sequentially consist of G0/G1, S, G2, and M [8]. If cell growth can be stagnated in the period from G0/G1 to S, an important rate-limiting period of cell cycle in tumors, cell mitosis would be inhibited [9]. Thus, inducing the stagnation of cell growth in tumors in G0/G1 has been considered as one of the important mechanisms in suppressing the cell proliferation in tumors, which has been confirmed in the various experimental studies on the tumor cells, such as prostatic cancer [1], breast cancer [2], and gastric cancer [3]. In this study, we found that at 12 h, 24 h and 48 h after the intervention of 5 mM metformin for oral squamous cell carcinoma SCC-4 cell and CAL-27 cell, gradual increases were identified in the ratio of cells in G0/G1 phase over time in a time-dependent manner. This indicated that metformin can affect the cell cycle of oral squamous cell carcinoma SCC-4 cell and CAL-27 cell to inhibit the cell proliferation.
Adenosine Monophosphate Activated Protein Kinase (AMPK), as a kind of “energy sensing enzyme” that exists in the eukaryotic cells in human, can participate in the energetic metabolism in the body [10]. AMPK will be activated when the ratio of AMP/ATP in cells is elevated under the lack of energy, which will further result in the activation of downstream signal pathway, thus affecting the stability of cells [11]. In this study, we found that after the intervention of metformin in concentration of 5 mM on the oral squamous cell carcinoma SCC-4 cell and CAL-27 cell, significant changes were observed in the total quantity of AMPKa, but significant increases were also identified in phosphorylation level of AMPKa (Thrl72) in a significant time-dependent manner; obvious decreases were observed in the expressions of mTOR and the downstream target molecules, such as p-mTOR (Ser2448), p-S6Kl (Thr389) and p-4E-BPl (Thr37/46) in a significant time-dependent manner. Metformin can significantly increase the phosphorylation level of AMPK on the locus of Thrl72 in the oral squamous cell carcinoma SCC-4 cell and CAL-27 cell, indicating that the inhibitory effect on cell proliferation of oral squamous cell carcinoma might be realized through the activation of AMPK signal pathway, but further studies are expected to clarify whether the inhibitory effect of metformin on the mTOR signal pathway in the cells of oral squamous cell carcinoma depends on the activation of AMPK signal pathway.
In conclusion, metformin can decrease the cell activity of oral squamous cell carcinoma SCC-4 cell and CAL-27 cell, inhibit the cell proliferation and clone formation in oral squamous cell carcinoma, and induce the stagnation of cell growth of oral squamous cell carcinoma in G0/G1. The mechanism of inhibition is correlated with the activation of AMPK signal pathway and the inhibition of mTOR signal pathway.
References
- Zhang J, Wang L, Xia P. Inhibition of epithelial-mesenchymal transition by metformin in prostate cancer cells and correlative mechanisms. J Shandong Univ (Health Sciences) 2013; 9: 210-214.
- Zhao Q, Ma J, Liu Y, Cai Q. Proliferation and apoptosis effect of combining metformin and adriamycin on breast cancer cells. Prog Mod Biomed 2013; 11: 201-208.
- Tong C, Wang W, Liang B. Experimental study of Metformin for inhibiting tumor angiogenesis and gastric cancer cell growth. China Med Herald 2015; 11: 128-131.
- Wang Y, Lu M, Zhou Y. Efficacy and safety of combination therapy with saxagliptin and metformin in patients with type 2 diabetes: a meta-analysis. Adv Drug React J 2012; 10: 124-128.
- Li M, Liu J, Hu W. Effect of metformin on apoptosis of renal cell carcinoma cells in vitro and its mechanisms. J South Med Univ 2011; 8: 224-228.
- Xu S, Yang Z, Jin P. Metformin suppresses the activity of ovarian cancer associated fibroblast through the downregulation of IL-6. Prog Obster Gynecol 2016; 11: 159-162.
- Zhang Y. The influence of metformin on the prognosis of type 2 diabetes with colorectal cancer and its potential mechanism. South Med Univ 2013.
- Wang R, Li Q, Wang J. Tumor cell cycle of long and short and apoptosis of more and less-The laws of tumor growth. J Fourth Military Med Univ 2012; 6: 334-338.
- Sun Y. Cyclind1 protein plays different roles in modulating chemoresponses in MCF7 and MDA-MB231 cells. Guangxi Med Univ 2014.
- Hu Y, Xu H, Wang Y. Effect of AMPK on energy metabolism of mice in exercise-induced and pathological cardiac hypertrophy. China Sport Sci 2014; 9: 153-154.
- Wang L, Guo C, Qu Q. Study on LKB1/AMPK/mTOR signal transduction pathway in endometrial cancer. Maternal Child Health Care China 2014; 4: 220-224.