ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2011) Volume 22, Issue 4
Role of homocysteine and lipoprotein (A) in atherosclerosis: An update
Devika Tayal #@, Binita Goswami*@, Bidhan Chandra Koner$ and V. Mallika*
1# Department of Biochemistry, Lady Hardinge Medical College and Associated Hospital, New Delhi, India
2* Department of Biochemistry, G B Pant Hospital, New Delhi, India
3$ Department of Biochemistry, Maulana Azad Medical College, and Associated Hospitals, New Delhi, India
4@ Both DT and BG have contributed equally for the paper.
- Corresponding Author:
- Binita Goswami
B 406 Panchsheel apartments Plot 9
Sector 10, Dwarka Delhi 110075 India
Accepted date: June 07 2011
Lipoprotein (a) and homocysteine are two important independent risk factors for coronary atherosclerosis. Lipoprotein (a) is a low-density lipoprotein -like particle formed by the as-sociation of the highly polymorphic glycosylated apolipoprotein (a) with apolipoprotein _B100. Homocysteine is formed as a by-product of the metabolism of amino acid methionine. The present review attempts to elaborate the roles of homocysteine and lipoprotein (a) in the etiopathogenesis of CAD and the inter relationship between these two established risk fac-tors. The literature was searched from the websites of the National Library of Medicine (http://www.ncbi.nlm.nih.gov/) and Pub Med Central, the U.S. National Library of Medi-cine’s digital archive of life sciences journal literature. The role of homocysteine and lipo-protein (a) has been extensively investigated during the past few decades. The outcomes have been varied with both favourable and unfavourable inferences. Certain researchers have also pointed towards interplay between these two risk factors at the molecular level. A better understanding of the interaction between the risk factors of CAD will help in better risk stratification and management of this disease.
Keywords
CAD, Lipoprotein (a); Homocysteine; Coronary Artery Disease; Atherosclerosis; Atherothrombosis.
Introduction
Cardiovascular disease (CVD) is the leading cause of death and disability in developed nations and is increasing rapidly in the developing world [1]. In more than 90% of instances, the cause of myocardial ischemia is reduction of coronary blood flow owing to atherosclerotic coronary arterial obstruction [2]. By the year 2020, it is estimated that CVD will surpass infectious diseases as the world's leading cause of death and disability [3]. Indians are pre-disposed to CVD due to a multitude of factors and present with an aggressive form of the disease. It has been pre-dicted that coronary artery disease might become the most prevalent disease in India by the year 2020 due to the rap-id changes in demography and life styles consequent to economic development [4]. Much research has gone into understanding the pathogenesis of CAD and atherosclero-sis; and the identification of the causative factors of this condition is imperative for tackling this pandemic.
Pathphysiology of atherosclerosis
The hallmark of atherosclerosis is the atherosclerotic plaque, which contains lipids (intracellular and extra cel-lular cholesterol and phospholipids), inflammatory cells (e.g., macrophages, T-cells), smooth muscle cells, con-nective tissue (e.g., collagen, glycosaminoglycans, elastic fibers), thrombi, and calcium deposits. The mechanism of atherosclerosis involves several highly interrelated proc-esses, including lipid disturbances, platelet activation, thrombosis, endothelial dysfunction, inflammation, oxida-tive stress, vascular smooth cell activation, altered matrix metabolism, remodeling, and genetic factors [5].
Risk factors
Risk factors play an important role in initiating and accel-erating the complex process of atherosclerosis. The major modifiable risk factors include elevated blood pressure, dyslipidemia, smoking, and diabetes mellitus. Unmodifi-able risk factors include age, gender, and family history/ genetics. A number of emerging risk factors for atherosclerosis have recently been proposed to help identify high-risk individuals. Newer risk factors include Lipoprotein (a) [Lp (a)], homocysteine, thrombotic risk factors and inflammatory risk factors [2]. In this present review we will discuss the role of Lp (a) and homocysteine and the inter-relation between these two risk factors and whether it leads to increased risk of atherothrombosis.
Lipoprotein (a) [Lp(a)]
Biochemistry
Lipoprotein (a) [Lp(a)] was identified by Berg in 1963[6]. It has been observed that individuals with low or null concentrations of plasma lipoprotein (a) manifest no deficiency syndrome or disease [7]. Lp (a) is a low-density lipoprotein (LDL)-like particle formed by the association of the highly polymorphic glycosylated apolipoprotein (a) (apo (a)) with apolipoprotein B100 (apo B100), the classic protein moiety of LDL [8]. Apo(a) is attached to Apo B100 through a single disulphide link between apo B100 Cys 3734 and apo(a) kringle IV type 9 Cys67[9]. The cysteine residue in apoB involved in the covalent bond between apoB and apo(a) is close to the postulated LDL receptor-binding region of apoB[10].
The human apo(a) gene is located in a gene cluster within 400 kb of genomic DNA on the telomeric region of chromosome 6 (6q26-27) [11]. The apo(a) gene belongs to a gene family that includes several similar sequences encoding prothrombin, tissue-type plasminogen activator (t- PA), urokinase A-chain, plasminogen, coagulation factor XII, macrophage stimulating factor, hepatocyte growth factor and other proteins and polypeptides of unclear function[12]. The apo (a) gene shares the highest homologies with the gene of the zymogen plasminogen. The plasminogen gene contains coding sequences for 5 different K domains (K1 to K5), and 2 of these are present in the apo (a) gene, K4 and K5. Due to size heterogeneity, apo (a) exhibits a genetic size polymorphism with apparent molecular weight of isoforms ranging from 300 to 800KDa [13, 14]. An inverse correlation between the number of kringle IV type 2 repeats in the apo (a) gene and Lp(a) plasma concentration exists[15]. The presence of apo (a) influences to a major extent metabolic and physicochemical properties of Lp(a) [16,17]. Although many studies have addressed differences between blacks and whites, population differences with regard to Lp(a) and apo(a) size are not limited to these groups. Differences in both Lp(a) levels and apo(a) size distribution have been noted among Asian populations. Thus, whereas East Asians have been reported to have a relatively narrow apo(a) size distribution and low Lp(a) levels, South Asians have higher mean Lp(a) levels. [18-23] Several polymorphisms in the apo(a) gene have been reported. Of these, a C/T variation in the promoter region of the apo(a) gene and a pentanucleotide repeat (TTTTAn) 1 kb upstream of the apo(a) gene have been studied .[24- 26]
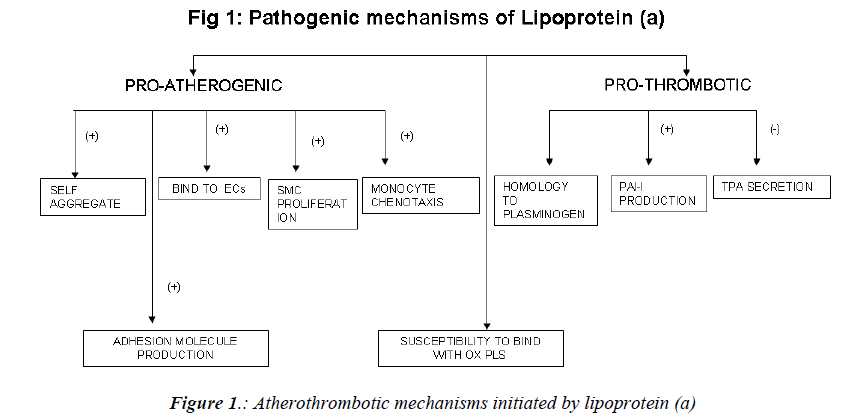
Mechanism of atherogenicity (Fig. 1)
Raised levels of Lp(a) are associated with an increased incidence of a variety of cardiovascular diseases, including silent coronary artery disease (CAD), acute myocardial infarction (AMI), asymptomatic carotid atherosclerosis, stroke, and peripheral artery occlusive disease (PAOD) [27,28]. The mechanism implicated in the atherogenicity of Lp (a) include.
a) tendency to self-aggregate and precipitate and a greater capacity to bind to glycosaminoglycans and other structures in the vascular wall. Lp(a) binds avidly to endothelial cells, macrophages, fibroblasts, and platelets, as well as to the subendothelial matrix; there, it promotes proliferation of vascular smooth muscle cells and chemotaxis of human monocytes [29-31]. Foam cell production is also enhanced by lp(a) [32].
b) Due to its unique structural homology to plasminogen, Lp(a) competes with plasminogen for binding to plasminogen receptors, fibrinogen, and fibrin[33].It also induces the production of plasminogen activator inhibitor 1 (the main inhibitor of the fibrinolytic system) and inhibits the secretion of tissue-plasminogen activator by endothelial cells [34,35]. It promotes generation of free radicals in monocytes [36].
c) In a series of studies, Witztum et al have demonstrated that a key oxidized phospholipid is preferentially associated with Lp(a) [37-39]. Proinflammatory, oxidized phospholipids are covalently bound to kringle V in apo(a) [37]. Another study suggests that oxidized phospholipids are physically associated with Lp(a) lipoprotein bound to lysine residues on isolated fragments of kringle V of apo(a) [40] and also in the lipid phase of Lp(a) lipoprotein. The presence of oxidized phospholipids in Lp (a), potentially being taken up by the vessel wall, accelerates development of atherosclerosis.
d) In an endothelial cell system, native Lp (a), via apo (a), stimulates the production of adhesion molecules such as intercellular adhesion molecule [41], vascular cell adhesion molecule-1[42], and E-selectin[42], as well as endothelin- 1[43] and I-309, a potent chemo attractant for monocytes[44]. In a vascular smooth muscle cell system, native Lp(a), and particularly apo(a), inhibits the proteolytic activation of transforming factor via a decrease in cell surface generation of plasmin, resulting in increased vascular smooth muscle cells proliferation[45].
Figure 1. Salivary Cortisol levels before and after vestibular stimulation in participants. (Data expressed are Mean ± SD) (*P value <0.05, **P<0.01, ***P<0.001). MV- Vestibular males, FVVestibular females, MC- control males, FC- Control females. D0- pre intervention score, D1- post intervention score.
Retrospective case-control studies have found a strong association between elevated Lp(a) levels and CVD. On the other hand, the results of prospective studies have been mixed. That plasma Lp(a) concentration is an inde-pendent risk factor for CVD in both men and women was confirmed by the Prospective Epidemiological Study of Myocardial Infarction (PRIME), which included 9133 French and Northern Irish men, aged 50 to 59 years at entry, without any manifestations of cardiovascular dis-ease. In these subjects, Lp (a) was significantly associated with CVD development and appeared to be a significant risk factor (P<0.0006) in the cohort as a whole. [46].
Studies have suggested an association between high levels of Lp (a) [>30 mg/dL]) and the presence and extent of coronary artery disease, premature coronary artery dis-ease, myocardial infarction, restenosis after balloon an-gioplasty, cerebrovascular disease, saphenous vein bypass graft disease, and cardiac allograft vasculopathy. This as-sociation has been documented in men and women and in white persons, African Americans, and Asian Indians [47-69].
However some studies have shown no association be-tween Lp (a) levels and vascular disease. In the Physi-cians' Health Study, which involved 14,916 predomi-nantly white, middle-aged men, serum Lp (a) level did not correlate with future risk for myocardial infarction or stroke[70,71]. In the Helsinki Heart Study, baseline Lp (a) levels were similar in patients who developed coronary events over 5 years of follow-up and in those who did not suffer from any such event [72]. A 5-year prospective follow-up study of 2156 French Canadian men showed that elevated Lp(a) level was not an independent risk fac-tor for coronary artery disease[73].In another study in-volving 140 African-American patients, plasma Lp(a) levels were found to be similar in those with and without coronary disease[74].
Indian perspective
Sharobeem et al conducted a study on 55 South Asian patients with ischaemic stroke (confirmed on computer-ised scan of the brain) and 85 controls and concluded that Lp(a) and the Apo B to AI ratio are associated with is-chemic stroke in South Asians [75]. Rajappa et al as-sessed the role of lp(a) in the prediction of CAD in south Indians with Diabetes Mellitus [76]. They confirmed that high concentrations of Lp(a) along with high prevalence of non insulin dependent Diabetes Mellitus (NIDDM), predispose Indians to atherosclerosis at an earlier age. We have also demonstrated the role of lp(a) as a risk factor in patients of acute myocardial infarction [77]. Singh et al concluded from their study that Lp(a) alone can correctly discriminate a CVD individual from a control subjects by 95% [78]. In few studies conducted in India higher mean levels of Lp(a) were observed in cases than controls- 12 to 41mg/dl in patients and 8 to 24 mg/dl in healthy con-trols [79-83]. Hence, lp(a) is a strong risk factor in Indi-ans.
Homocysteine
Biochemistry
Homocysteine "hypothesis of arteriosclerosis" was first proposed by McCully in 1969, when he observed prema-ture atherothrombosis of the peripheral, coronary, and cerebral vasculature in children with homocystinuria, an inborn error in methionine metabolism [84]. Homocys-teine, a sulphur-containing amino acid, is an intermediate formed during the catabolism of the essential dietary amino acid methionine. Approximately 80% of plasma homocysteine is protein bound. Free and protein bound homocysteine and its disulfides are globally referred to as total homocysteine (tHcy) [85-87]. Homocysteine is me-tabolized by two pathways: vitamin B6-dependent trans-sulfuration and vitamin B12-dependent and folate-dependent remethylation [88]. In 1976 Wilcken and Wil-cken first showed that patients with coronary heart dis-ease have elevated concentrations of plasma cysteine – homocysteine disulphide after an oral methionine load [89].
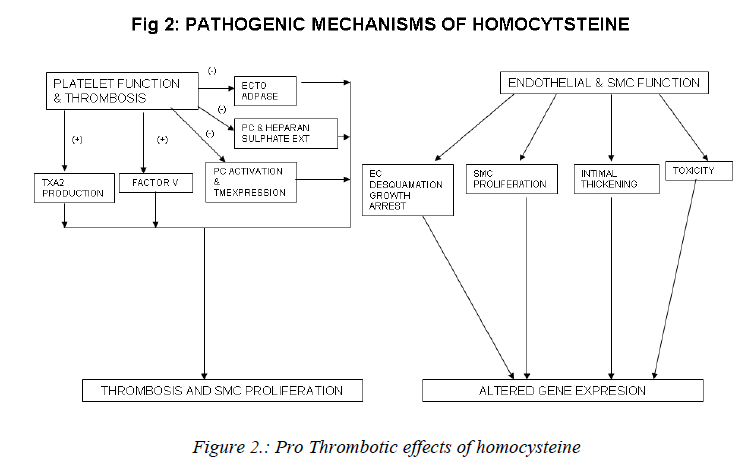
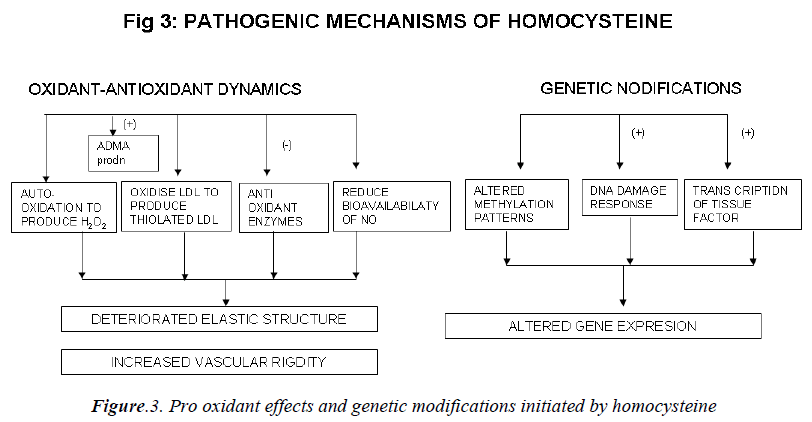
Mechanisms involved in the athero-thrombotic effects of homocysteine (Figs. 2,3)
The mechanisms by which hyperhomocysteinemia might contribute to atherogenesis and thrombosis are incom-pletely understood and various theories have been pro-pounded for the same. Some of them are highlighted un-der the following subheads.
a) Effects on platelet function and thrombosis: The vas-cular endothelium maintains a non-thrombogenic surface with the help of antithrombin III and thrombomodulin-protein C mediated anticoagulant pathways [90, 91]; syn-thesis of plasminogen Activators [92-94] and the inhibi-tion of platelet activation by ecto-ADPase, prostacyclin and nitric oxide [92]. The endothelium also synthesises clotting factors and maintains the balance between proco-agulant and anticoagulant mechanisms, which is vital to the maintenance of vascular haemostasis. Homocysteine may upset this balance and predispose to thrombogenesis via stimulation of procoagulant factors and promotion of coagulation in the absence of thrombin [95]. Homocys-teine stimulates platelet generation of thromboxane A2 which is a vaso-constrictor and proaggregant. Hyper ho-mocysteinemia causes: activation of factor V and inter-feres with protein C activation and thrombomodulin ex-pression [96], interferes with the generation and bio-availability of prostacyclin and the expression of anti-coagulant substance heparan sulphate on vessel wall [97]. It also inhibits the activity of ecto-ADPase[98]. Platelet activation due to hyperhomocystenemia results in throm-bosis and smooth muscle proliferation and is therefore important in the pathogenesis of atherothrombosis [99, 100].
b) Effects on endothelial and smooth muscle function:
Vascular intimal smooth muscle proliferation with subse-quent formation of extracellular matrix collagen is an in-tegral component of atherosclerosis [100]. This process may be directly stimulated by homocysteine or may be secondary to the mitogenic effect of endothelial and/or platelet-derived growth factors released by homocysteine-induced endothelial cell damage. The signal pathway for the stimulation of DNA synthesis in smooth muscle cells by homocysteine involves cyclins ,cyclin-dependent kinases, neutrophil docking protein complex CD11b/ CD18 [101] and endothelial expression of monocyte chemoattractant protein-1[102]. Homocysteine causes endothelial cell desquamation, smooth muscle cell prolif-eration and intimal thickening [103]. High concentrations of homocysteine are also toxic to endothelial cells in vitro [104]. Homocysteine inhibits DNA synthesis in vascular endothelial cells and causes growth arrest at the G1 phase of cell cycle [105]. Pathological concentrations of homo-cysteine also increase the interaction between neutrophils and endothelial cells. This results in neutrophil migration across the endothelium with concurrent damage and de-tachment of endothelial cells [106]. Further, homocys-teine induces mitogenesis in vascular smooth muscle cells by stimulating MAP kinase signal transduction pathway and by the induction of C-fos and C-myb genes [107].
c) Effect on the oxidant antioxidant dynamics:
Homocysteine can undergo auto-oxidation forming hy-drogen peroxide (H2O2) as a by product. H2O2 can then lyse endothelial cells [108]. Homocysteine can oxidize LDL as well as combine with LDL to form thiolated LDL which is taken up by foam cells much faster [109]. Ho-mocysteine has been shown to inhibit intracellular anti-oxidant enzymes, including glutathione peroxidase thus decreasing the cell’s ability to neutralize oxidant radicals [110]. Peroxynitrite is also increased in peripheral blood vessels during hyperhomocysteinemia. Peroxynitrite is known to activate poly ADP-ribose polymerase (PARP), and activation of PARP may be an important mediator of vascular dysfunction. Homocysteine reduces bioavailabil-ity of nitric oxide (NO) [111] and causes deterioration of the elastic structure of the arterial wall through alteration in metalloproteinase activity. It may also increase vascu-lar rigidity by augmenting breakdown of elastin in vascu-lar cells [112]. Liao et al have demonstrated that plasma Hcy level has a significant, negative correlation with apoA-I concentration in human CAD [13]. Hyperhomo-cysteinemia may produce vascular dysfunction and pro-mote oxidative stress by increasing levels of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthase (NOS). ADMA also inhibits basal relaxation in cerebral blood vessels [114].
d) Effects at the genetic level:
DNA methylation is a critical component of epigenetic regulation of gene expression, particularly during devel-opment. DNA hypomethylation is induced by increase in homocysteine levels. Thus global or selective DNA me-thylation may contribute to alterations in gene expression and vascular changes during hyperhomocysteinemia [115]. Homocysteine at high concentration also increases the transcription and activity of tissue factor [116]. Ho-mocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotox-ins. DNA strand breaks and associated activation of PARP and NAD (nicotinamide adenine dinucleotide) de-pletion occur rapidly after exposure to homocysteine and precede mitochondrial dysfunction, oxidative stress and caspase activation [117].
Studies evaluating the role of homocysteine in cad
Despite huge literature available, a uniform and widely acceptable view regarding the role of homocysteine has not been established. Medical fraternity is still witnessing differing opinions regarding need to treat hyperhomocys-teinemia. The association between homocysteine levels and CAD has been observed more in retrospective studies as compared to prospective studies. Several studies have reported that CAD is associated with high homocysteine levels. In the Framingham Heart Study, and in women enrolled in the Atherosclerosis Risk in Communities (ARIC) study, homocysteine levels were higher in adults with CHD [118,119]. In the British Regional Heart Study, homocysteine levels were significantly (P=0.004) higher in patients with stroke [120]. The frequency of hospitali-zation for cardiovascular disease was correlated with baseline homocysteine levels, particularly in the oldest age group (hospitalization-rate ratio per 5 μmol/L increase in homocysteine: 1.29 versus 1.10; probability value for interaction, 0.02) [121].
The Homocysteine Studies Collaboration combined evi-dence from 12 prospective and 18 retrospective studies conducted during 1966 to 1999. A total of 5,073 CAD events and 1,113 stroke events were observed among 16,786 healthy individuals. The results showed that a 25% increase in the serum homocysteine concentration (an increase of approximately 3 μmol/l) is associated with a 49% higher risk of ischemic heart disease (IHD) in the retrospective studies [126].
Homocysteine lowering, achieved after the introduction of the folate food fortification programme in North Amer-ica, was accompanied by a decline in cardiovascular risk especially of stroke. Although the initial clinical trials suggested that homocysteine-lowering treatment with folates and B vitamins induces coronary plaque regres-sion, this finding was not confirmed by more recent clini-cal studies [127].
Several large-scale studies totaling nearly 50,000 subjects are currently under way in the U.S., Canada, and Europe-WENBIT (the Western Norway B-vitamin Intervention Trial), SEARCH (Study of Effectiveness of Additional Reduction in Cholesterol and Homocysteine) trial, PA-CIFIC (Prevention with a Combined Inhibitor and Folate in Coronary Heart Disease) trial, VITATOPS (Vitamins to Prevent Stroke) trial, and others. One will have to await the results from these trial data before finally confirming or refuting the homocysteine hypothesis in atherothrom-botic vascular disease [128].
The results of the Physicians' Health Study, the Nurses' Health Study, the European Concerted Action Study, and the Hordaland Homocysteine Study all support the valid-ity of the homocysteine theory of arteriosclerosis [129].Meta-analysis of published studies suggests that elevation of homocysteine is a causal factor in athero-genesis; such studies predicted that lowering homocys-teine concentrations would be estimated to benefit 15–40% of the population by preventing vascular dis-ease[130].
Humphrey et al concluded from their recent meta analysis in 2008 that homocysteine has a positive predictive value in CAD risk assessment. Meta-analysis yielded a com-bined risk ratio for coronary events of 1.18 (95% confi-dence interval, 1.10-1.26) for each increase of 5 micro-mol/L in homocysteine level. The association between homocysteine and CHD was similar when analyzed by sex, length of follow-up, outcome, study quality, and study design. They concluded that each increase of 5 mi-cromol/L in homocysteine level increases the risk of CHD events by approximately 20%, independently of tradi-tional CHD risk factors [131].
However, these positive findings need to be interpreted with caution as some studies have not confirmed the ho-mocysteine theory of atherosclerosis. There is evidence to suggest that homocysteine is released from damaged vas-cular tissue after myocardial infarction and stroke. Hence, it is often considered that increased homocysteine levels may be an effect rather than cause of CAD It is proposed that during the repair of damaged tissue, there is an in-creased demand for DNA. These repair processes require the methylation of DNA, RNA and proteins - reactions that lead to the generation of homocysteine as the end point of methylation. Cleophas et al concluded from their meta-analysis that although the data analysed till date demonstrate a strong association between homocysteine and CAD, they do not support the hypothesis that the relationship is causative. Homocysteine may not be as harm-ful to the heart as it appears to be, but it may be an impor-tant indicator for an unhealthy lifestyle, making it an im-portant variable in assessing patients for CAD risk [132].
Brilakis et al , concluded from their study on 500 patients, that multivariate analysis which included age, gender, smoking, LDL, HDL, Lp(a), apo A1, and apo B revealed no independent association between quartile of homocys-teine and odds ratio (OR) for CAD[133].
Indian perspective
Due to a predominant vegetarian diet, Indians are defi-cient in vitamin B12 and consequently have higher serum levels of homocysteine [134]. There are studies which support as well as refute the role of this elevated level of homocysteine in the pathogenesis of CAD among Indians. Puri et al evaluated the role of homocysteine in patients of premature CAD. They found that plasma homocysteine was a significant (OR 6.05) independent risk factor for young CAD patients in India [135]. Bhargav et al ob-served that Hcy is significantly higher in patients of vas-cular diseases as compared to normal controls [136]. Abraham et al and Rao et al also demonstrated signifi-cantly higher homocysteine levels in patients of CAD as compared to healthy controls [137, 138].
Pandey et al concluded from their study conducted on 137 consecutive women who attended a health care program that no positive correlation between elevated hcy levels and CAD was demonstrated in spite of a large percentage of persons showing elevated homocysteine levels [139]. Deepa et al conducted a study analysing the homocysteine levels in Indian diabetic males. They concluded that ho-mocysteine did not play a role in CAD risk assessment in diabetic males [140]. There is a need for further popula-tion based studies to evaluate the atherogenecity of ho-mocysteine in the Indian scenario.
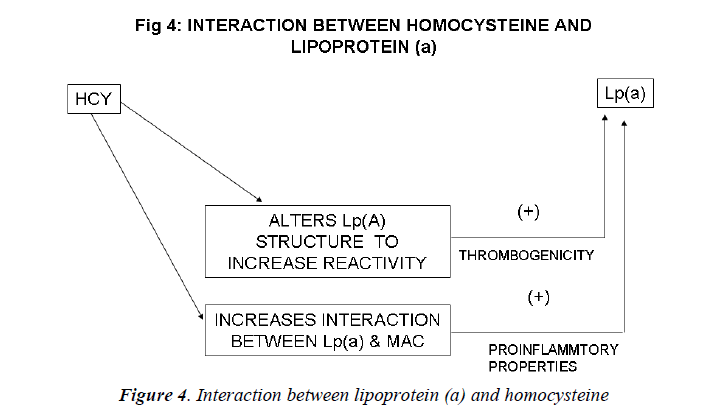
Inter-relation between Lp (a) and homocysteine (Figs. 4, 5)
There is a growing body of evidence that atherosclerosis is the final outcome of the complex interplay of a multi-tude of risk factors interacting through various inter-twined molecular mechanisms. Evidence for an interac-tion between Lp(a) and other established or emerging risk factors for cardiovascular disease, such as LDL choles-terol, high-density lipoprotein cholesterol, and homocys-teine, have been found[141-143].
Harpel et al demonstrated an inter-relation between ho-mocysteine and Lp(a) concentrations. He found that ho-mocysteine alters the intact Lp (a) particle so as to in-crease the reactivity of the plasminogen-like apolipopro-tein (a) portion of the molecule. Homocysteine, at con-centrations as low as 8micromol/L, significantly increases the affinity of Lp (a) for fibrin. Homocysteine induces a 20-fold increase in the affinity between Lp (a) and plas-min-treated fibrin and a 4-fold increase with untreated fibrin. Thus, the largest effect of homocysteine was on the binding of Lp(a) to plasmin modified fibrin. The dissocia-tion constant (Kd) for the interaction between Lp (a) and plasmin-treated fibrin was 0.18 nM in the presence of homocysteine and 3.67 nM in its absence. The effect of homocysteine on increasing Lp(a) binding to fibrin was proportional to the concentration of the sulfhydryl com-pound added[144]. This study illustrated the remarkable susceptibility of Lp(a) to sulfhydryl compounds of physiological significance, including homocysteine, glu-tathione, and cysteine. In the presence of these com-pounds, the binding of Lp (a) to fibrin is significantly en-hanced. The binding of homocysteine-treated Lp (a) to plasmin-modified fibrin was found to be specific since homocysteine did not increase the binding of Lp (a) to other immobilized proteins.
Lp (a) may be altered by homocysteine in individuals with hyperhomocysteinemia, by glutathione in the micro-environment of the thrombus, or by other reductive mech-anisms that remain to be established. Limited reduction may produce a reorientation of apo (a) kringle(s) with exposure of additional binding sites not previously acces-sible to the fibrin surface. Lp(a) particles with enhanced fibrin affinity may prove to be an important link between thrombosis, atherogenesis, and sulfhydryl compound me-tabolism[145].
Western blot analysis of Lp(a) documented that homocys-teine caused a concentration dependent decrease in the amount of Lp(a) migrating at the position of the intact particle, and an increase in free apoB-100 and apo(a). This indicated that homocysteine had reduced the putative disulfide bond between apoB- 100 and apo(a) . In pre-liminary immunoblot ligand studies, it was found that fibrin degradation products bind to the free apo(a) pro-duced by homocysteine, suggesting that reduced apo(a) loses functional activity[145].
Foody et al carried out a cross-sectional study [146] for baseline characteristics of all patients referred to the Cleveland Clinic Foundation (CCF) Preventive Cardiol-ogy and Rehabilitation Program (PCRP) from 1996 to 1998. It was found that when both high homocysteine and lipoprotein (a) were present as risk factors in women, the associated risk was greater than what would be expected if the risk due to these two factors were simply added up. This cross-sectional analysis demonstrates that Lp (a) and tHcy interact to increase the risk of CAD in women. Higher serum concentrations of both factors increase the CAD risk by nearly 5 times. This theory is consistent with the suggestion that apo (a) is the atherogenic moiety of Lp(a), as noted in transgenic mouse models[147].
Ghorbanihaghjo et al observed an association between homocysteine and Lp(a) in the pathogenesis of retinal arteriosclerosis[148]. Nardulli et al demonstrated by im-munochemical, ligand-binding and plasminogen activa-tion studies, that homocysteine modifies the structure and function of Lp (a) in human plasma by reducing the apo(a)/apoB disulfide bond causing the appearance of free apo(a) with high affinity for fibrin that inhibits plasmino-gen binding and plasmin formation . Lp (a) and homocys-teine may therefore be considered to be acting in a con-sortium in order to increase the risk of atherothrombosis [149].
Sotiriou et al also evaluated the interaction between ho-mocysteine and Lp(a).They found that proatherogenic factor, homocysteine, can augment the interaction be-tween Lp(a) and Mac-1 integrin, potentiating the proin-flammatory action of Lp(a). Lp (a) and homocysteine synergize to increase the risk for coronary artery disease: when both risk factors are present, the associated risk is greater than what would be expected if these two risk fac-tors were acting independently. Their data showing that homocysteine enhances the proinflammatory action of Lp(a) may provide a novel clue as to how the interaction of these two risk factors increases cardiovascular risk[150].
The interaction of Lp(a)/apo(a) with the ternary complex is attributed in part to lysine binding sites within the kringle 4-like domains of apo(a). Thus, the interaction of apo(a) with Mac-1 shares similarities with the antifibri-nolytic activity of apo(a). The fact that smaller isoforms of Lp(a) have been recognized as a risk factor for coro-nary heart disease independent of plasma Lp(a) concentra-tions has been partially attributed to the fact that smaller apo(a) isoforms bind more avidly to fibrin and are more effective in the inhibition of plasmin formation. Sotiriou’s work describes a novel proinflammatory function of Lp(a)/apo(a) that directly interacts with Mac-1 and medi-ates inflammatory cell recruitment as well as concomitant up-regulation of the prothrombotic tissue factor. The pro-inflammatory mechanism of Lp(a) potentiated in the pres-ence of homocysteine improves our understanding of the proatherogenic potential of these emerging cardiovascular risk factors.
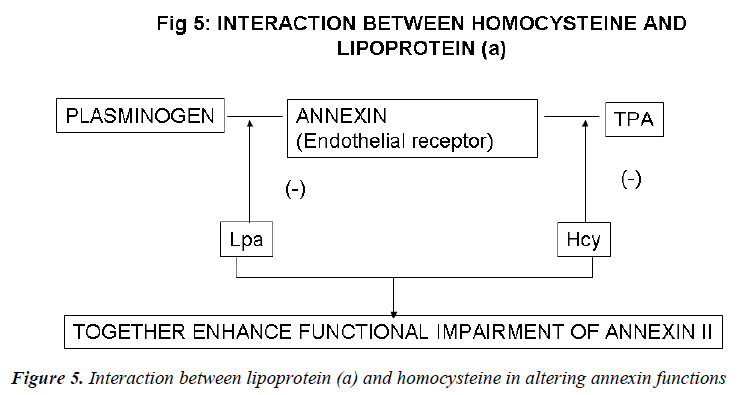
Recent evidence indicates a potential role for the plas-min/plasminogen activator system in the prevention of atherosclerotic vascular disease. Fibrin deposition is a common histologic feature of the tissues of mice that are genetically deficient in one or more key components of the fibrinolytic system. Cell surface receptors may sup-port fibrinolytic surveillance in both intravascular and extravascular locations by stimulating the efficiency of plasmin generation and by protecting plasmin from its inhibitors. In vitro studies suggest that the endothelial cell receptor, annexin II, which independently binds both plasminogen and t-PA, could play a key role in the proc-ess. Binding of plasminogen to annexin II is specifically inhibited in the presence of excess concentrations of the atherogenic LDL-like particle Lp(a). Similarly, t-PA binding to annexin II is blocked by homocysteine, a sulf-hydryl-containing amino acid that is associated with atherogenesis and that directly derivatizes the t-PA bind-ing domain of annexin II. The presence of both the risk factors, thus, accentuates the functional impairment of annexin II [151]. Hopkins et al [152] analyzed plasma Lp(a), lipids, and other coronary risk factors in a case-control study of men and women with premature athero-sclerosis. The relative risk for CAD in patients with Lp(a) values >40 mg/dL was 2.9. An Lp(a) level >40 mg/dL and high tHcy resulted in an OR of 32, with a CI between 6.5 and 155.
These findings support the hypothesis that tHcy and Lp (a) interact to increase the risk of CAD. A high tHcy level may act in concert with a high Lp (a) level to promote atherosclerosis and or vascular disease. These data pro-vide an interesting hypothesis-generating finding regard-ing the differential interactive effects of 2 emerging car-diovascular risk factors and may have important implica-tions for the prevention and treatment of CAD in select high-risk populations.
In contrast, in a prospective analysis of young men with premature peripheral atherosclerosis (N=95), Valentine et al [153] reported no significant interaction between Lp(a) and tHcy in defining risk of CAD. Other studies have also reported non existence of any synergism between homo-cysteine and Lp (a) [154,155] In one study, high serum total homocysteine (tHcy) and lipoprotein (a) [Lp(a)] lev-els were found to be independent risk factors for cardio-vascular disease [154]. An investigator in another study found a positive correlation between the levels of homo-cysteine and those of Lp(a) (Spermann's rho = 0.54, p < 0.001) but their effect on coronary heart disease was found to be independent [155].
The above mentioned studies highlight the need for fur-ther studies to validate or refute Harper’s hypothesis that suggested a synergestic interaction between the two risk factors -homocysteine and Lp (a) in atherothrombosis. It may provide an important link between thrombosis, ath-erogenesis and sulphhydryl compound metabolism.
Perspective
Homocysteine and Lipoprotein (a) are two well known risk factors for atherothrombosis. To the best of our knowledge, ours is the first broad review that attempts to evaluate the inter relationship between these two risk fac-tors. The association between these two can be further potentiated by extensive meta analysis of the studies that have attempted to evaluate this intricate relationship. A randomised control trial that evaluates the effect of medi-cation on homocysteine and Lp (a) and their further effect in ameliorating or attenuating CAD risk , is the need of the hour.
Last but definitely, not the least, such elaborate research and studies should be helpful to the general population and this can be accomplished by the development of drugs that can manage the multiple aetiologies effectively in a comprehensive manner.
Conclusion
The current understanding of atherosclerosis is as a chronic inflammatory process, developing in response to some metabolic disorders (dyslipidemia, insulin resis-tance), infections and environmental processes that initi-ate and promote lesion development to the point of acute thrombotic complications. The growing knowledge about the interplay between homocysteine and lipoprotein (a) has many far reaching implications. Their synergism calls for more stringent treatment modalities in subjects having high homocysteine as well as lipoprotein (a) levels.
Understanding the complex interplay between the various risk factors will be very beneficial in devising lifestyle modifications and pharmacological interventions to deal with CAD, a disease that has assumed epidemic propor-tions.
Abbreviations:
ADMA – Dimethyl arginine
AMI- Acute Myocardial Infarction
CI- Confidence Interval
CAD- Coronary Artery Disease
CVD- Cardiovascular Disease
EC- Endothelial Cell
HDL- High Density Lipoprotein
LDL- Low Density Lipoprotein
NAD- Nicotinamide Adenine Dinucleotide
NO- Nitric Oxide
NOS- Nitric Oxide Synthase
OR- Odds Ratio
PAOD- Peripheral Artery Occlusive Disease
PARP- Poly ADP Ribose Polymerase
PC- Protein C
SMC- Smooth Muscle Cell
TM- Thrombomodulin
TPA- Tissue Plasminogen Activator
TXA2- Thromboxane A2
References
- Fleming R M. Angina and coronary ischemia are the results of coronary regional blood flow differences. J Amer Coll Angiol 2003; 1:127-142.
- Mallika V, Goswami B, Medha R. Atherosclerosis pathophysiology and role of novel risk factors: A clini-cobiochemical Perspective. Angiology. 2007; 58(5):513-522.
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet1997; 349:1269-1276.
- Mohan V, Deepa R, Rani SS, Premlatha G. Prevalence of coronary artery disease and its relationship to lipids in a selected population in south India: CUPS No 5. J. Am Coll Cardiol. 2001; 38:682-687.
- Libby P. Inflammation in atherosclerosis. Nature 2002; 420: 868?874.
- Berg, K. 1963. A new serum type system, the lp sys-tem. Acta Pathol. Microbiol. Scand.; 59: 369-382.
- G. Lippi G and Guidi G. Lipoprotein (a): from ances-tral benefit to modern pathogen? Q J M 2000; 93: 75-84.
- Berg K. Lp(a) lipoprotein: an overview. Chem Phys Lipids1994; 67?68:9?16.
- Guevara J Jr, Valentinova NV, Garcia O, Gotto AM, Yang CY, Legal S, Gaubatz J, Sparrow JT. Interaction of apolipoprotein[a] with apolipoproteinB-100 Cys3734 region in lipoprotein[a] is confirmed immu-nochemically. J Protein Chem 1996; 15:17?25.
- Callow MJ, Rubin EM. Site-specific mutagenesis dem-onstrates that cysteine 4326 of apolipoprotein B is re-quired for covalent linkage with apolipoprotein (a) in vivo. J Biol Chem1995; 270: 23914?23917.
- Magnaghi P, Citterio E, Malgaretti N, Acquati F, Otto-lenghi S, Taramelli R. Molecular characterisation of the human apo(a)-plasminogen gene family clustered on the telomeric region of chromosome 6 (6q26-27). Hum Mol Genet 1994; 3:437?42.
- Yamamura Y, Yamashiro K, Tsuruoka N, Nakazato H, Tsujimura A, Yamaguchi N. Molecular cloning of a novel brain-specific serine protease with a kringle-like structure and three scavenger receptor cysteine-rich motifs. Biochem Biophys Res Commun.1997; 239: 386?92.
- Byrne CD, Schwartz K, Meer K, Cheng JF, Lawn RM. The human apolipoprotein (a) /plasminogen gene clus-ter contains a novel homologue transcribed in liver. Ar-terioscler Thromb.1994; 14: 534?41.
- Mbewu AD and Durrington PN. Lipoprotein (a): struc-ture and possible involvement in thrombogenesis and atherogenesis. Atherosclerosis 1990; 85: 1-14.
- Kraft HG, Lingenhel A, Kochl S, et al. Apolipoprotein (a) kringle IV repeat number predicts risk for coronary heart disease. Arterioscler Thromb Vasc Biol 1996; 16: 713-719.
- Hobbs HH, White AL. Lipoprotein (a): Intrigues and insights. Curr Opin Lipidol.1999; 10: 225?236.
- Berglund L. Diet and drug therapy for lipoprotein (a). Curr Opin Lipidol 1995; 6: 48-56.
- Geethanjali FS, Luthra K, Lingenhel A, Kanagasaba-Pathy AS, Jacob J, Srivastava LM, Vasisht S, Kraft HG, Utermann G. Analysis of the apo(a) size polymor-phism in Asian Indian populations: association with Lp(a) concentration and coronary heart disease. Ather-osclerosis 2003; 169: 121?130
- Hughes K, Aw TC, Kuperan P, Choo M. Central obe-sity, insulin resistance, syndrome X, lipoprotein (a) and cardiovascular risk in Indians, Malays, and Chinese in Singapore. J Epidemiol Community Health 1997; 51: 394?399.
- Hoogeveen RC, Gambhir JK, Gambhir DS, Kimball KT, Ghazzaly K, Gaubatz JW, Vaduganathan M, Rao RS, Koschinsky M, Morrisett JD. Evaluation of Lp(a) and other independent risk factors for CHD in Asian Indians and their USA counterparts. J Lipid Res.2001; 42: 631?638. 21. Utermann G. Genetic architecture and evolution of the lipoprotein (a) trait. Curr Opin Lipidol 1999; 10: 133?141.
- Kraft HG, Lingenhel A, Pang RWC, Delport R, Trommsdorf M, Vermaak H, Janus ED, Utermann G. Frequency distributions of apolipoprotein (a) kringle IV repeat alleles and their effects on lipoprotein (a) levels in Caucasian, Asian, and African populations: the dis-tribution of null alleles is non-random. Eur J Hum Genet 1996; 4: 74?87.
- Lars BL and Ramakrishnan R. Lipoprotein (a): An Elu-sive Cardiovascular Risk Factor. Arteriosclerosis, Thrombosis, and Vascular Biology 2004;24:2219-2226.
- Trommsdorff M, Köchl S, Lingenhel A, Kronenberg F, Delport R, Vermaak H, Lemming L, Klausen C, Faergeman O, Utermann G, Kraft H-G. A pentanucleo-tide repeat polymorphism in the 5' control region of the apolipoprotein (a) gene is associated with lipoprotein (a) plasma concentrations in Caucasians. J Clin In-vest.1995; 96: 150?157.
- Mooser V, Mancini FP, Bopp S, Pethö-Schramm A, Guerra R, Boerwinkle E, Müller H-J, Hobbs HH. Se-quence polymorphisms in the apo(a) gene associated with specific levels of Lp(a) in plasma. Hum Mol Genet 1995; 4: 173?181.
- Rubin J, Pearson TA, Reed RG, Berglund L. A fluores-cence-based, non-radioactive method for efficient de-tection of the pentanucleotide repeats (TTTTA) n polymorphism in the apolipoprotein (a) gene. Clin Chem.2001; 47: 1758?1762.
- Rodriguez CR, Seman LJ, Ordovas JM, Jenner J, Genest MS Jr, Wilson PW, Schaefer EJ. Lipoprotein (a) and coronary heart disease. Chem Phys Lipids 1994; Lippi G, Guidi G. Biochemical risk factors and pa-tient's outcome: the case of lipoprotein (a). Clin Chim Acta. 1999; 280:59?71.
- Pillarisetti S, Paka L, Obunike JC, Berglund L, Gold-berg IJ. Subendothelial retention of lipoprotein (a): evi-dence that reduced heparan sulfate promotes lipopro-tein binding to subendothelial matrix. J Clin In-vest.1997; 100:867-874.
- Grainger DJ, Kemp PR, Liu AC, Lawn RM, Metcalfe JC. Activation of transforming growth factor-beta is in-hibited in transgenic apolipoprotein (a) mice. Nature 1994; 370:460-462.
- Poon M, Zhang X, Dunsky KG, Taubman MB, Harpel PC. Apolipoprotein (a) induces monocyte chemotactic activity in human vascular endothelial cells. Circulation 1997; 96:2514-2519.
- Naruszewicz M, Selinger E, Davignon J. Oxidative modification of lipoprotein (a) and the effect of ß-carotene. Metabolism 1992; 41:1215-24.
- Loscalzo J. Lipoprotein (a): a unique risk factor for atherothrombotic disease. Arteriosclerosis 1990; 10: 672-679.
- Li XN, Grenett HE, Benza RL, et al. Genotype-specific transcriptional regulation of PAI-1 expression by hy-pertriglyceridemic VLDL and Lp(a) in cultured human endothelial cells. Arterioscler Thromb Vasc Biol.1997; 17:3215-3223.
- Levin EG, Miles LA, Fless GM, et al. Lipoproteins inhibit the secretion of tissue plasminogen activator from human endothelial cells. Arterioscler Thromb.1994; 14: 438-442.
- Riis HP, Kharazmi A, Jauhianen M, Ehnholm C. In-duction of oxygen free radical generation in human monocytes by lipoprotein (a). Eur J Clin Invest.1994; 24:497-9.
- Edelstein C, Pfaffinger D, Hinman J, Miller E, Lipkind G, Tsimikas S, Bergmark C, Getz GS, Witztum JL, Scanu AM. Lysine-phosphatidylcholine adducts in kringle V impart unique immunological and potential pro-inflammatory properties to human apolipoprotein (a). J Biol Chem.2003; 278: 52841?52847.
- Silaste ML, Rantala M, Alfthan G, Aro A, Witztum JL, Kesäniemi YA, Hörkkö S. Changes in dietary fat intake alter plasma levels of oxidized low-density lipoprotein and lipoprotein (a). Arterioscler Thromb Vasc Biol 2004; 24: 498?503.
- Tsimikas S, Bergmark C, Beyer RW, Patel R, Pattison J, Miller E, Juliano J, Witztum JL. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syn-dromes. J Am Coll Cardiol 2003; 41: 360?370.
- Edelstein C, Pfaffinger D, Hinman J, et al. Lysine-phosphatidylcholine adducts in Kringle V impart unique immunological and potential pro-inflammatory properties to human apolipoprotein (a). J Biol Chem 2003; 278: 52841-52847.
- Takami S, Yamashita S, Kihara S, Ishigami M, Take-mura K, Kume N, Kita T, and Matsuzawa Y. Lipopro-tein (a) enhances the expression of intercellular adhe-sion molecule-1 in cultured human umbilical vein en-dothelial cells. Circulation 1998; 97: 721-728.
- Allen S, Khan S, Tam S, Koschinsky M, Taylor P, and Yacoub M. Expression of adhesion molecules by Lp(a): a potential novel mechanism for its atherogenicity .FASEB J.1998;12:1765-1776.
- Berge K E, Djurovic S, Muller H J, Alestrom P, and Berg K. Studies on effects of Lp(a) lipoprotein on gene expression in endothelial cells in vitro).Clin. Genet 1997; 52: 314-325.
- Haque N S, Zhang X, French D L, Li J, Poon M, Fallon JT, Gabel BR, Taubman, MB, Koschinsky M, and Har-pel P. 2000. Chemokine I-309 Is the Principal Mono-cyte Chemoattractant Induced by Apolipoprotein (a) in Human Vascular Endothelial Cells. Circulation 2000; 102:786-792.
- Grainge, DL, Kemp PR, Liu AC, Lawn RM, and Met-calfe JC. Activation of transforming growth factor-ß is inhibited in transgenic apolipoprotein (a) mice. Nature 1994; 370:460-462.
- Luc G, Bard JM, Arveiler D, et al. Lipoprotein (a) as a predictor of coronary heart disease: the PRIME Study. Atherosclerosis 2002; 163: 377?384
- Bostom AG, Gagnon DR, Cupples LA, Wilson PW, Jenner JL, Ordovas JM, et al. A prospective investiga-tion of elevated lipoprotein (a) detected by electropho-resis and cardiovascular disease in women. The Fram-ingham Heart Study Circulation 1994; 90:1688-95.
- Schaefer EJ, Lamon-Fava S, Jenner JL, McNamara JR, Ordovas JM, Davis CE, et al. Lipoprotein (a) levels and risk of coronary heart disease in men. The Lipid Re-search Clinics Coronary Primary Prevention Trial. JAMA 1994; 271:999-1003.
- Rosengren A, Wilhelmsen L, Eriksson E, Risberg B, Wedel H. Lipoprotein (a) and coronary heart disease: a prospective case?control study in a general population sample of middle aged men. BMJ 1990; 301:1248-51.
- Genest J Jr, Jenner JL, McNamara JR, Ordovas JM, Silverman SR, Wilson PW, et al. Prevalence of lipopro-tein (a) [Lp(a)] excess in coronary artery disease. Am J Cardiol 1991; 67:1039-1045.
- Budde T, Fechtrup C, Bosenberg E, Vielhauer C, Enbergs A, Schulte H, et al. Plasma Lp(a) levels corre-late with number, severity, and length-extension of coronary lesions in male patients undergoing coronary arteriography for clinically suspected coronary athero-sclerosis. Arterioscler Thromb.1994; 14:1730-6.
- Dahlen GH, Guyton JR, Attar M, Farmer JA, Kautz JA, Gotto AM Jr. Association of levels of lipoprotein Lp(a), plasma lipids, and other lipoproteins with coro-nary artery disease documented by angiography. Circu-lation 1986; 74:758-65.
- Bostom AG, Cupples LA, Jenner JL, Ordovas JM, Se-man LJ, Wilson PW, et al. Elevated plasma lipoprotein (a) and coronary heart disease in men aged 55 years and younger. A prospective study. JAMA 1996; 276:544-8. Sandkamp M, Funke H, Schulte H, Kohler E, Assmann G. Lipoprotein (a) is an independent risk factor for myocardial infarction at a young age. Clin Chem 1990; 36:20-3.
- Durrington PN, Ishola M, Hunt L, Arrol S, Bhatnagar D. Apolipoproteins(a), AI, and B and parental history in men with early onset ischaemic heart disease. Lancet 1988; 1(8594):1070-3.
- Genest J Jr, McNamara JR, Ordovas JM, Jenner JL, Silberman SR, Anderson KM, et al. Lipoprotein cho-lesterol, apolipoprotein A-I and B and lipoprotein (a) abnormalities in men with premature coronary artery disease. J Am Coll Cardiol.1992; 19:792-802.
- Cremer P, Nagel D, Labrot B, Mann H, Muche R, Elster H, et al. Lipoprotein Lp(a) as predictor of myo-cardial infarction in comparison to fibrinogen, LDL cholesterol and other risk factors: results from the pro-spective Gottingen Risk Incidence and Prevalence Study (GRIPS). Eur J Clin Invest. 1994; 24:444-53.
- Rhoads GG, Dahlen G, Berg K, Morton NE, Dannenberg AL. Lp(a) lipoprotein as a risk factor for myocardial infarction. JAMA.1986; 256:2540-4.
- Desmarais RL, Sarembock IJ, Ayers CR, Vernon SM, Powers ER, Gimple LW. Elevated serum lipoprotein (a) is a risk factor for clinical recurrence after coronary balloon angioplasty. Circulation 1995; 91:1403-9.
- Zenker G, Koltringer P, Bone G, Niedorkorn K, Pfeif-fer K, Jurgen G. Lipoprotein (a) as a strong indicator for cerebrovascular disease. Stroke 1989; 17:942-5.
- Chambless LE, Shahar E, Sharrett AR, Heiss G, Wijnberg L, Paton CC, et al. Association of transient ischemic attack/stroke symptoms assessed by standard-ized questionnaire and algorithm with cerebrovascular risk factors and carotid artery wall thickness. The ARIC Study, 1987-1989. Am J Epidemiol. 1996; 144:857-66.
- Van Kooten F, van Krimpen J, Dippel DW, Hooger-brugge N, Koudstaal PJ. Lipoprotein (a) in patients with acute cerebral ischemia. Stroke 1996; 27:1231-5.
- Solymoss BC, Marcil M, Wesolowska E, Lesperance J, Pelletier LC, Campeau L. Risk factors of venous aorto-coronary bypass graft disease noted at late symptom-directed angiographic study. Can J Cardiol.1993; 9:80-4.
- Barbir M, Kushwaha S, Hart B, Macken H, Thompson GR, Mitchell A, et al. Lipoprotein (a) and accelerated coronary artery disease in cardiac transplant recipients. Lancet 1992; 340:1500-2.
- Orth-Gomer K, Mittleman MA, Schenk-Gustafsson K, Wamala SP, Eriksson M, Belkic K, et al. Lipoprotein (a) as a determinant of coronary heart disease in young women Circulation 1997; 95: 329-334.
- Schreiner PJ, Heiss G, Tyroler HA, Morrisett JD, Davis CE, Smith R. Race and gender differences in the asso-ciation of Lp(a) with carotid artery wall thickness. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol.1996; 16: 471-478.
- Schreiner PJ, Chambless LE, Brown SA, Watson RL, Toole J, Heiss G. Lipoprotein (a) as a correlate of stroke and transient ischemic attack prevalence in a bi-racial cohort: the ARIC Study. Atherosclerosis Risk in Communities. Ann Epidemiol.1994; 4:351-9.
- Dhawan J. Coronary heart disease risks in Asian Indi-ans. Curr Opin Lipidol. 1996; 7:196-8.
- Sunayama S, Daida H, Mokuno H, Miyano H, Yokoi H, Lee YJ, et al. Lack of increased coronary atheroscle-rotic risk due to elevated lipoprotein (a) in women > or = 55 years of age. Circulation 1996; 94:1263-8.
- Ridker PM, Hennekens CH, Stampfer MJ. A prospec-tive study of lipoprotein (a) and the risk of myocardial infarction. JAMA 1993; 270:2195-9.
- Ridker PM, Stampfer MJ, Hennekens CH. Plasma con-centration of lipoprotein (a) and the risk of future stroke. JAMA.1995; 273:1269-73.
- Jauhiainen M, Koskinen P, Ehnholm C, Frick MH, Manttari M, Manninen V, et al. Lipoprotein (a) and coronary heart disease risk: a nested case?control study of the Helsinki Heart Study participants. Atherosclero-sis 1991; 89:59-67.
- Cantin B, Gagnon F, Moorjani S, Despres JP, La-marche B, Lupien PJ, et al. Is lipoprotein (a) an inde-pendent risk factor for ischemic heart disease in men? The Quebec Cardiovascular Study. J Am Coll Cardiol 1998; 31:519-25.
- Moliterno DJ, Jokinen EV, Miserez AR, Lange RA, Willard JE, Boerwinkle E, et al. No association be-tween plasma lipoprotein (a) concentrations and the presence or absence of coronary atherosclerosis in Af-rican-Americans. Arterioscler Thromb Vasc Biol 1995; 15:850-5.
- Sharobeem KM, Patel JV, Ritch AE, Lip GY, Gill PS, Hughes EA. Elevated lipoprotein (a) and apolipopro-tein B to AI ratio in South Asian patients with ischae-mic stroke. Int J Clin Pract. 2007; 61(11):1824-8.
- Rajappa M, Sridhar MG, Balachander J, Sethuraman KR. Lipoprotein (a) and comprehensive lipid tetrad in-dex as a marker for coronary artery disease in NIDDM patients in South India. Clin Chim Acta. 2006; 372(1-2):70-5.
- Goswami B, Rajappa M, Mallika V, Singh B, Ray PC, Kumar S. Inflammation and Dyslipidemia: a possible interplay between established risk factors in north In-dian males with coronary artery disease. Cardiovascu-lar Journal of South Africa 2010; 21(2): 107-12.
- Singh S, Dwivedi S, Melkani GC, Rani C, Gaur SP, Mandal SK, Mahua J. Lipoprotein(a) and coronary heart disease in Indian population. JAPI 1999; 47(12): 1157-60.
- Gambhir JK, Harsimrut K, Gambhir DS and Prabhu KM. Lipoprotein (a) as an independent risk factor for Coronary artery disease in patients below 40 years of age. IHJ.2000; 52: 411-415
- Enas EA and Senthilkumar A. Role of Lipoprotien(a): Reality and Relevance. In: Gambhir, D. (ed.) Cardiol-ogy Update 2000. CSI, New Delhi p 8-25.
- Gambhi, JK, Gambhir DS and Morreset J. Lipoprotein (a) in normal Indian subjects and patients with coronary artery disease. IHJ. 1998; 50: 563.
- Gupta R, Kastia S, Rastogi S, Kaul V, Nagar R and Enas EA. Lipoprotein(a) in Coronary Heart Disease: A Case-Control Study. IHJ.2002; 52: 407- 410.
- Mohan V, Deepa R and Rema M. Lipoprotein (a) and coronary artery disease in Indians. JAPI 1998; 46, 979-980.
- McCully KS. Vascular pathology of homocysteinemia. Implications for the pathogenesis of arteriosclerosis. Am J Pathol1969; 56:111-128
- 85. Coltaneo M. Hyperhomocysteinemia. Atherosclero-sis and Thrombosis. Thromb Haemost.1999; 81:165-76.
- Haynes WG. Hyperhomocysteinemia V, vascular func-tion and atherosclerosis: effect of vitamins. Cardiovas-cular Drugs and Therapy 2002; 16:391-399.
- Rasmussen K, Moller J. Total homocysteine measure-ment in clinical practice. Ann Clin Biochem.2000; 37: 627-648.
- Finkelstein JD. Methionine metabolism in mammals. J Nutri Biochem.1990;1: 228-37.
- Wilcken DEL, Wilcken B. The pathogenesis of coro-nary artery disease. A possible role for methionine me-tabolism. J Clin Invest 1976; 57: 1079-82.
- Ziegler DM. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem.1985; 54: 305?29.
- Esmon CT, Owen WG. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of pro-tein C. Proc Natl Acad Sci USA 1981; 78: 2249?52.
- Rodgers GM. Hemostatic properties of normal and perturbed vascular cells. FASEB J 1988; 2: 116?23.
- Hajjar K. Assembly of the fibrinolytic system on endo-thelial cells. In: Haber E, Braunwald E, eds. Throm-bolysis: Basic Contributions and Clinical Progress. Mosby-Year Book 1991: 27?32.
- Hajjar KA. Homocysteine-induced modulation of tissue plasminogen activator binding to its endothelial cell membrane receptor. J Clin Invest 1993; 91: 2873?9.
- Rodgers GM, Kane WH. Activation of endogenous factor V by a homocysteine-induced vascular endothe-lial cell activator. J Clin Invest 1986; 77: 1909?16.
- Lentz SR, Sadler JE. Inhibition of thrombomodulin surface expression and protein C activation by the thrombogenic agent homocysteine. J Clin Invest 1991; 88:1906-1914.
- Nishinaga M, Ozawa T, Shimado K. Homocysteine, a thrombogenic agent, suppresses anticoagulant heparin suphate expression in cultured procine aortic endothe-lial cells. J Clin Invest 1993; 92: 1381-1386.
- Broekman MJ, Haijar KA, Marcus AJ, Lev E, Islam N, Safier LB, Flreosbach J. Homocysteine inhibits ecto ADPase activity of human umbilical vein endothelial cells. Blood 1994; 84 (Suppl):77-80.
- Ross R. The pathogenesis of atherosclerosis-an update. N Engl J Med 1986; 314: 488?500.
- Ross R. The pathogenesis of atherosclerosis: a perspec-tive for the 1990s. Nature 1993; 362 (6423): 801?9.
- Dudman NP, Temple SE, Guo XW, Fu W, Perry MA. Homocysteine enhances neutrophil-endothelial interac-tions in both cultured human cells and rats in vivo. Circ Res 1999;84:409?16.
- Poddar R, Sivasubramaniam N, Robinson K, Jacobsen DW. Homocysteine modulates the expression of a spe-cific cytokine (Monocyte chemoattractant protein-1) in human aortic endothelial cells. Circulation 1997; 96: I-286.
- Harker LA, Ross R, Slichter SJ, Scolt CR. Homo-cystine ? induced arterosclerosis: the role of endothelial cell injury and platelet response in its genesis. J Clin Invest.1996; 58: 731-41.
- Wall RT, Harlan JM, Harker LA, Striker GE. Homo-cysteine induced endothelial cell injury in vivo: A re-view. Thromb Res.1980; 18:113-121.
- Wang H, Yoshizumi M, Lai K. Inhibition of growth and p21ras methylation in vascular endothelial cells by homocysteine but not cysteine. J Biol Chem 1997; 272: 25380-25385.
- Dudman NPB, Temple SE, Guo XW, Fu WY, Perry MA. Homocysteine enhances neutrophil endothelial in-teractions in vivo. Circ Res 1999; 84: 409-416.
- Haynes WG. Hyperhomocysteinemia Vascular function and atherosclerosis: Effect of vitamins. Cardiovascular Drugs and therapy 2002; 16:391-99.
- Starkebaum G, Harlan JM. Endothelial cell injury due to copper catalysed hydrogen peroxide generation from homocysteine. J Clin Invest 1993; 77:1370-1376.
- Heinecke JW, Rosen H, Suzuki LA, Chait A. The role of sulphur containing amino acids in superoxide pro-duction and modification of low density lipoprotein in arterial smooth muscle cells. J Biol Chem 1987; 262: 10098-10103.
- Upchurch GR, Welch GN, Fabian AJ. Homocysteine decreases bioavailable Nitric oxide by a mechanism in-volving glutathione peroxidase. J Biol Chem 1997; 272: 17012-17017.
- Emsley AM, Jeremy JV, Gomes GN, Angelini GD, Plane F. Investigation of the inhibitory effects of ho-mocysteine and copper on nitric oxide mediated relaxa-tion of rat isolated aorta. Brit J Pharmacol.1999; 126: 1034-1040.
- Rolland PH, Friggi A, Barlatier A, Piquet P, Latrille V, Faye MM. Hyperhomocysteinemia induced vascular damage in pig circulation. Circulation 1995; 91: 1161-1174.
- Liao D, Tan H, Hui R, Li Z, Jiang X, Gaubatz J, Yang F, Durante W et al,. Hyperhomocysteinemia Decreases Circulating High-Density Lipoprotein by Inhibiting Apolipoprotein A-I Protein Synthesis and Enhancing HDL Cholesterol Clearance. Circulation Res. 2006; 99:598-603.
- Boger RH. The emergent role of asymmetric dimethy-largine as a novel cardiovascular risk factor. Cardio Vasc Res 2003; 59: 824-833.
- Hiltunen MO, Via-Herttual S. 2000. DNA methylation smooth muscle cells and atherogenesis. Arterioscler Thromb Vasc Biol; 20: 1557-1564.
- Fryer RH, Wilson BD, Gubler DB, Fitzgerald LA, Rodgers GM. Homocysteine, a risk factor for prema-ture vascular disease and thrombosis, induces tissue factor activity in endothelial cells. Arterioscler & Thrombosis 1993; 13: 1327?33.
- Krumkan II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, Matson MR. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to cxcitotoxicity. J Neurosci 2000; 20(18): 6920-6926.
- Bostom AG, Silbershatz H, Rosenberg IH, et al. Non-fasting plasma total homocysteine levels and all-cause and cardiovascular disease mortality in elderly Fram-ingham men and women. Arch Intern Med.1999; 159: 1077?1080.
- Folsom AR, Nieto FJ, McGovern PG, et al. Prospective study of coronary heart disease incidence in relation to fasting total homocysteine, related genetic polymor-phisms, and B vitamins: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 1998; 98: 204?210.
- Perry IJ, Refsum H, Morris RW, et al. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet 1995; 346: 1395?1398.
- Nurk E, Tell GS, Vollset SE, et al. Plasma total homo-cysteine and hospitalizations for cardiovascular dis-ease: the Hordaland Homocysteine Study. Arch Intern Med.2002; 162: 1374?1381.
- Boushey CJ, Beresford SAA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 1995; 274: 1049-57.
- Stampfer MJ, Malinow MR, Willett WC, Newcomer LM, Upson D, Tiswer PV, Hennekens CH. A prospec-tive study of plasma homocysteine and risk of myocar-dial infarction in US physicians. JAMA 1992; 268: 877-81.
- Montalescot G, Ankri A, Chadefau X, Vekemans B. Plasma homocysteine and the extent of atherosclerosis in patients with coronary artery disease. Int J Cardiol 1997; 60:295-300.
- Nygard O, Nordvehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Eng J Med 1997; 337: 230-236.
- Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG. MTHFR Studies Collaboration Group MTHFR 677C T polymorphism and risk of coronary heart diseases meta-analysis. JAMA 2002; 288:2023-2031.
- Antoniades C, Antonopoulos A.S, Tousoulis D, Mari-nou Kand Stefanadis C. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. European Heart Journal 2009; 30 (1):6-15.
- Kaul S, Zadeh AA and Prediman K. Shah PK. Homo-cysteine Hypothesis for Atherothrombotic Cardiovas-cular Disease .Not Validated. Am Coll Cardiol 2006; 48:914-923.
- McCully KS. Homocysteine, folate, vitamin B6 and cardiovascular disease. JAMA 1998; 279:392?3.
- Wald DS, Law M, Morris JK. Homocysteine and car-diovascular disease: evidence of causality from a meta-analysis. Br Med J 2002; 325: 1202-1209.
- Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease in-cidence: a systematic review and meta-analysis. Mayo Clin Proc.2008; 83(11):1200-2.
- Cleophas TJ, et al. Homocysteine, a risk factor for cor-onary artery disease or not? A meta-analysis. J Am Coll Cardiol 2000; 86:1005-9.
- Brilakis ES, McConnell JP, Ballman KV, Klee GG, Berger PB. Lack of association between plasma homo-cysteine and angiographic coronary artery disease in the era of fortification of cereal grain flour with folic acid. Atherosclerosis 2002; 165(2):375-81.
- Lakshmi AV, Maniprabha C and Krishna TP. Plasma homocysteine level in relation to folate and vitamin B6 status in apparently normal men. Asia Pacific J Clin Nutr 2001; 10(3): 194?196.
- Puri A, Gupta OK, Dwivedi RN, Bharadwaj RP, Na-rain VS, Singh S. Homocysteine and lipid levels in young patients with coronary artery disease. JAPI 2003; 51:681-5.
- Bhargava S, Parakh R and Srivastava LM. Studies on homocysteine demonstrating its significance as a possi-ble tool for differential diagnosis in occlusive vascular disease. IJCB 2004; 19(1):76-78.
- Abraham R, John MJ, Dhanoa CJ. Raised serum homo-cysteine levels in patients of coronary artery disease and the effect of vitamin B12 and folate on its concen-tration. IJCB 2006; 21 (1): 95-100.
- Rao H, Govindaraju V, Manjunath CN. Risk prediction ? homocysteine in coronary heart disease. IJCB 2007; 22 (1):18-21.
- Pandey SN, Vaidya AD, Vaidya RA, Talwalkar S. Hy-perhomocysteinemia as a cardiovascular risk factor in Indian women: determinants and directionality. JAPI 2006; 54: 769-774.
- Deepa R, Velmurugan K, Saravanan G, Karkuzhali K, Dwarakanath V, Mohan V. Absence of Association be-tween Serum Homocysteine Levels and Coronary Ar-tery Disease in South Indian Males. IHJ 2001; 53: 44?47.
- Maher VM, Brown BG, Marcovina SM, Hillger LA, Zhao XQ, Albers JJ. Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein (a). JAMA.1995; 274: 1771?1774
- Cantin B, Gagnon F, Moorjani S, Despres JP, La-marche B, Lupien PJ, Dagenais GR. Is lipoprotein (a) an independent risk factor for ischemic heart disease in men? The Quebec Cardiovascular Study. J Am Coll Cardiol.1998; 31: 519?525
- Von Eckardstein A, Schulte H, Cullen P, Assman G. Lipoprotein (a) further increases the risk of coronary events in men with high global cardiovascular risk. J Am Coll Cardiol. 2000; 201; 37: 434?439.
- Harpel PC, Chang VT, Wolfgangborth E. Homocys-teine and other sulfhydryl compounds enhance the binding of lipoprotein (a) to fibrin: A potential bio-chemical link between thrombosis, atherogenesis, and sulflhydryl compound metabolism. Proc. NatL. Acad. Sci. 1992; 89:10193-10197.
- Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B & Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med 1991; 324: 1149-1155.
- Foody JM, Milberg JA, Robinson K, Pearce GL, Jacobsen DW, Sprecher DL. Homocysteine and Lipo-protein (a) Interact to Increase CAD Risk in Young Men and Women. Arterioscl Thromb Vasc Biol.2000; 20: 493-98.
- Lawn RM, Wade DP, Hammer RE, Chiesa G, Verstuyft JG,Rubin EM. Atherogenesis in transgenic mice expressing human apolipoprotein (a). Nature 1992; 360: 670-672.
- Ghorbanihaghjo A, Javadzadeh A, Argani H, Nezami N, Rashtchizadeh N, Rafeey M, Rohbaninoubar M,2 and Rahimi-Ardabili B. Lipoprotein(a), homocysteine, and retinal arteriosclerosis. Mol Vis 2008 ; 14: 1692?1697.
- Nardulli M, Durlach V, Pepe G, Anglés-Cano E. Mechanism for the homocysteine-enhanced antifibri-nolytic potential of lipoprotein (a) in human plasma. Thromb Haemost. 2005; 94 (1): 75-81.
- Sotiriou SN, Orlova VV, Al-Fakhri N, Ihanus E, Econ-omopoulou M, Isermann B et al. Lipoprotein (a) in ath-erosclerotic plaques recruits inflammatory cells through interaction with Mac-1 integrin. FASEB J 2006; 20:559-561. Hajjar KA, Jacovina AT. Modulation of annexin II by homocysteine: implications for atherothrombosis. J In-vestig Med 1998; 46 (8): 364-369.
- Hopkins PN, Wu LL, Hunt SC, James BC, Vincent GM, Williams RR. Lipoprotein (a) interactions with li-pid and nonlipid risk factors in early familial coronary artery disease. Arterioscler Thromb Vasc Biol 1997; 17:2783?2792.
- Valentine RJ, Kaplan HS, Green R, Jacobsen DW, My-ers SI, Clagett GP. Lipoprotein (a), homocysteine, and hypercoagulable states in young men with premature peripheral atherosclerosis: a prospective, controlled analysis. J Vasc Surg. 1996; 23: 53-61.
- Guven A, Inanc F, Kilinc M and Ekerbicer H. Plasma homocysteine and lipoprotein (a) levels in Turkish pa-tients with metabolic syndrome. Heart and Vessels 2005; 20 (6): 290-295.
- Laraqui A, Bennouar N, Meggouh F, Allami A, El Kadiri N, Benkouka F et al. Homocysteine, lipopro-tein (a): risk factors for coronary heart disease. Ann Bi-ol Clin 2002; 60 (5): 549-557.