ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 3
Role of stromal vascular fraction in severe burn wound healing process
1Department of Breast and Thyroid Surgery, Binzhou Central Hospital, Binzhou City, Shandong Province, China
2Department of Neurology, Binzhou Central Hospital, Binzhou city, Shandong Province, China
3Department of Bone and Joint, Binzhou Central Hospital, Binzhou City, Shandong Province, China
4Department of Burn, Binzhou People’s Hospital, Binzhou City, Shandong Province, China
- *Corresponding Author:
- Hongwei Yan
Department of Breast and Thyroid Surgery
Binzhou Central Hospital, China
Accepted on August 03, 2016
Objective: To determine role of Stromal Vascular Fraction (SVF) in wound healing process of hot water induced burns.
Materials and methods: Wound healing is characterized as the reclamation of the congruity of living tissue and is an incorporated reaction of a few cell types to damage. SVF was obtained from inguinal fat of rats. Artificial burns were induced in rats with help of hot water. VEGF, PCNA analysis performed in different groups with histological evaluation of deep burns and fibrosis.
Results: On day 10, aggravation intensity was watched to be extraordinarily expanded in the control set, while it was diminished in the treatment group and the contrast between the groups was measurably critical. The Vascular endothelial growth factor (VEGF) values were statistically significant between the therapy and control groups at 3 and 10 days. Statistically significant differences were observed among day 3 and other days in both the groups.
Conclusion: The wound healing benefit is associated with a more efficient vascular network. This topical therapy provides a potential novel therapy for the treatment of various wound based atrophies.
Keywords
Wound healing, Stem cell, angiogenesis, VEGF.
Introduction
Skin harm can happen as a delayed consequence of abrasions, cuts and scratched zones to fluctuating degrees of reality. If not repaired, any break of the skin may exchange off its impediment limit and reveal the body's tissues to microbial infections and mechanical mischief. The recovering of cutaneous wounds obliges complex interchanges between the dermal and epidermal cells, the extracellular matrix (ECM), also, the nervous and vascular parts of the hurt besides, including skin [1,2]. Recovery from cutaneous injuries takes place in specific phases: incendiary reactions, proliferation and remodelling. Different types of cells, chemokines and hormones are involved in these interconnected phases, in which the injured part is repaired and the integrity of the tissue is restored [3].
Healing of wounds takes place in a sequence of stages, namely, inflammation, hemostasis, aggravation, scar tissue development and the resulting remodelling [4]. Mesenchymal stem cells (MSCs) are non-hematopoietic, adherent multipotent stromal cells that can potentially differentiate into the antecedent cells for bone, ligament, muscle, tendon, stroma and fat tissues [5,6]. A stem cell is described by its capacity to undergo self-restoration, its capacity for multi-lineage separation and frame terminally separated cells. Stem cells used for regenerative therapeutic applications should preferably satisfy these criteria: (1) they must be found in abundance (millions to billions of cells). (2) It must be possible to collect them minimally intrusively. (3) It must be possible to separate them along various cell lineage pathways in a reproducible way. (4) It must be possible to transplant them securely and adequately to an autologous or an allogeneic host [7].
Superficial injuries may recover without enlisting undifferentiated cells; but repair of deeper tissue damage or wounds requires immature stem cells with mesenchymal and natural origin. However, also inhabitant tissue-particular undeveloped cells all through entirely unexpected all around deliberate repair falls vital for compensation of tissue [5,8] Senescent cells that are incapable of replicating themselves, which may influence adjacent typical cells, are involved in the complex set of changes that influence cell replication. Intracellular changes, for example, oxidative damage to DNA and changes in mitochondrial effectiveness and in cell layers, in maturing cells and tissues have been studied [9].
Adipose tissue, like bone marrow, is derived from the mesenchyme and consists of a uniform stroma that is effectively separated. Given this, adipose tissue may represent a source of stem cells that could have broad impacts [10]. The cellular reactions to injuries are primarily facilitated by mesenchymal stem cells that produce paracrine indicators or signals and induce antecedent hematopoietic stem cells, follicle stem cells and epithelial tissue to separate into the inhabitants of tissues. These cell types have specific roles in every stage of repair, and they expedite inflammation [3]. In the present study, the use of stromal vascular fractionate to treat wounds caused by burns was investigated. In vivo and in vitro testing was used to confirm the efficacy of stromal cells in healing burn wounds.
Materials and Method
Experimental animals
Twenty male adult Wistar rats weighing between 187 and 380 g were used in this study. The rats were monitored within a standard laboratory environment. All the rats were fed with standard laboratory diet before and during the study.
Chemicals for anesthesia
The rats were anesthetized using the combination of ketamine hydrochloride [+/- Ketamine HCl, Sigma Aldrich (50 mg/kg)] and xylazine hydrochloride [Xylazine hydrochloride ≥ 99% | Sigma-Aldrich, (5 mg/kg)] intraperitoneally. Early postoperative and five days post-burn pain management was performed by adding 2 mg/ml Acetaminophen (Acetaminophen, Sigma-Aldrich) in rats drinking water [11,12].
Stromal vascular fractionate preparation
Both inguinal regions of the rats were clipped and cleaned with sterile 10% povidone iodine. Inguinal fat pads of 20 rats were removed (two for each rat) and 1 cm3 adipose tissue was excised from each inguinal fat pad on both sides. The incisions were closed by 3/0 silk sutures (Figure 1). We collected inguinal cells from the same rats to make a master batch of (stromal vascular fractionate cells) SVFs. The extracted fat tissues were washed with phosphate buffer solution (PBS, Sigma-Aldrich, China) to evacuate the polluting cells and red platelets. Then the tissues were minced using tissue separating scissors (Fine). The separated tissues were incubated in a slow shaking water bath at 37°C for 120 minutes with 0.1% collagenase (collagenase from Clostridium histolyticum C0130, Sigma-Aldrich, China). The tissues were then removed, and the samples were further washed with PBS. The cell suspension was centrifuged twice at 1300 rpm (260 xg) for 8 minutes. The supernatant, which consisted of mature adipocytes, was decanted. The precipitate, which had collected at the bottom, was filtered using a 100 mm mesh filter, providing SVFs [13-15]. The number of viable cells was assessed by adding trypan blue to the SVFs and counting them using a Thoma slide. There was approximately 4×106 viable cells/ml [16].
Deep partial thickness burn
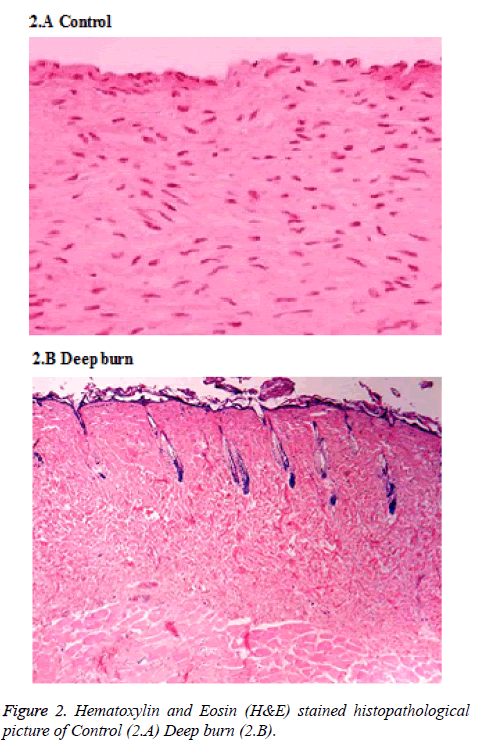
The rats were primed by clipping the hair on their back skin and cleaned with sterile 10% povidone iodine underneath general anesthesia. Burns were performed with the pet bottle opening area of 7 cm2 with a radius of 1.5 cm. The burn injury was made by exposing the back skin of the rat to hot water 80°C in the bottle. Deep partial thickness burns were found and confirmed by histopathologically (Figure 2). Open management without the application of topical antibiotics treated burn wounds. Created burn wound area was approximately 2% of total body surface area (TBSA) which was calculated by the formula defined by Gilpin [17]. On the exact SVFs harvestation, the burn injury were made by applying 80°C hot water for 30 sec on the back skin of the rats and then they were randomized into therapy and control groups of 10 rats each.
Vascular endothelial growth factor (VEGF) expression
Vascular endothelial growth factor expression was assessed using 400X magnification for the presence VEGF positive cells and the staining intensity. The percentage of VEGF positive was scored as follows: (1) <33 % of the cells, (2) 33-66% of the cells and (3) >66% of the cells. The staining intensity of VEGF expression was graded as (1) weak, (2) moderate and (3) strong. The sum of these scores was calculated as the VEGF index [18,19].
Proliferating cell nuclear antigen analysis (PCNA)
Nuclear brown staining was assessed as positive distinct by proliferating cell nuclear antigens antibody in the tissue section. PCNA was appraised by counting 100 cells under the 400X magnification. The PCNA index was determined by the ratio of the number of positive nuclei/100 nuclei [20,21].
Histopathology evaluation of the burn sites
Biopsies were taken sequentially from each quadrant of 7 cm2 circular burn under general anesthesia at 3, 7, 10 and 14 days, respectively. About 5 × 10 × 5 mm of the tissues were carried by biopsy and were embedded in paraffin blocks and were stained using hematoxylin-eosin.
Statistical analysis
Data are expressed as mean ± standard error. Differences between means were assessed using two tailed unpaired Student’s t test for two independent groups and paired Student’s t test for two repeated measures. When there were more than two repeated measures, we used repeated measures ANOVA with the post hoc Hochberg procedure. Statistical significance was accepted at p<0.05.
Results
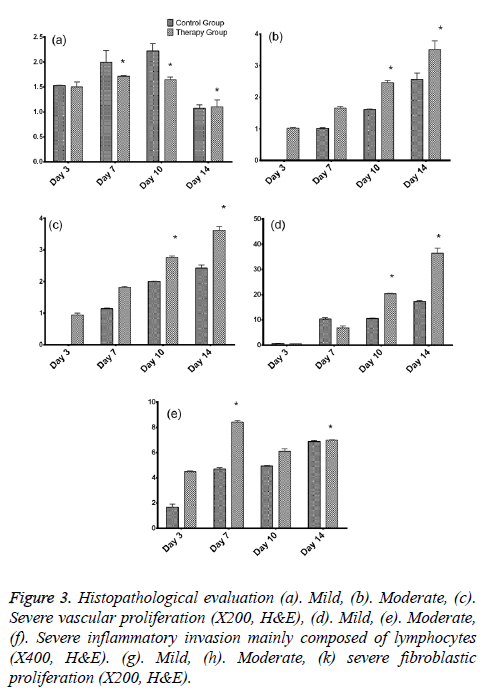
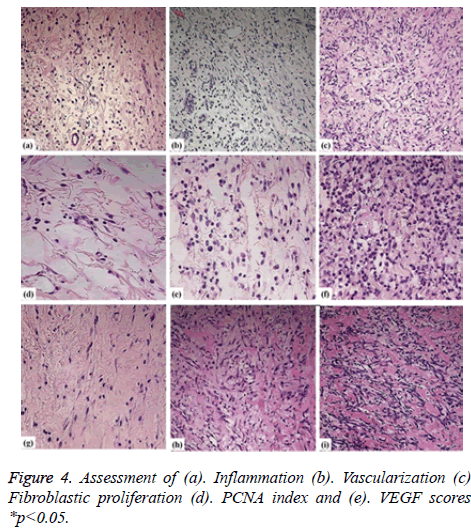
As per the dissected tissue samples, both gatherings had very nearly break even with intense inflammation at post burn on the third and seventh days. On day 10, exacerbation power was viewed to be exceptionally extended in the control group, while it was decreased in the treatment group and the difference between the groups was quantifiably discriminating. The histopathological evaluation was carried and depicted as shown in Figure 3. While on the on day 14, inflammation was decreased in the control group compared to the therapy group. Statistically significant differences between both the groups were observed about the vascularization at 3, 10, and 14 days. The fibroblastic proliferation showed significant difference between 3 and 14 days, whereas there was no significant difference at 7 and 10 days. The variance analysis of distribution of fibroblastic proliferation relative to the biopsy days in control group revealed that there were differences at 3, 7 and 10 days. Stromal cells including the endothelial cells and fibroblasts showed positive for the PCNA and the same was tested to find the PCNA index. The comparison of the values showed statistically significant differences on 3, 10 and 14 days (Figures 4a-4d). Vascular endothelial growth factor expression was seen in stromal cells including endothelial cells, inflammatory cells and fibroblasts. The VEGF values were statistically significant between the therapy and control groups at 3 and 10 days. The therapy group had a VEGF expression score that was almost twice the value of the control group on day 3. The VEGF expression scores continued to be higher in the therapy group than in the control group until day 10. On day 14, both groups had similar VEGF expression scores (Figure 4e). The VEGF expression score was double compared to the control group on the day 3. Increased expression scores of VEGF sustained in the therapy group than in the control group till day 10. Statistically significant differences were observed among day 3 and other days in both the groups.
Discussion
The injury recuperating process in adult humans can be partitioned into three covering stages: inflammation, multiplication and rebuilding. These techniques include the facilitated endeavours of keratinocytes, fibroblasts, endothelial cells, macrophages and platelets, directed by a mind boggling flagging system of particles. Cell relocation or migration is a crossroads in skin wound healing. Subsequently, the examination of variables that effect dermal fibroblast and epidermal keratinocyte development may help target medications for upgraded cutaneous damage healing [22]. Thermal injuries demonstrate a characteristic cutaneous injury pattern, which is divided into three zones based on blood perfusion and tissue viability: zone of coagulation, zone of stasis, and zone of hyperemia. The final zone of hyperemia is an area of intense vasodilatation and inflammation that contains viable tissue and is not usually at risk of progression to necrosis [23].
The proliferative phase is characterized by the formation of granulation tissue, which is a pink, soft, highly vascularized platform for tissue formation. Granulation tissue is largely a product of two cell types: fibroblasts and vascular endothelial cells. Fibroblasts are evident at the wound site within 2-5 days and become the predominant cell type after the first week [24]. Angiogenesis and the development of granulation tissue take place simultaneously during the injury recuperation process [25].
It was further recommended that dermal fibroblast relocation be then ended as keratinocytes relocate to launch reepithelialisation, after which a further influx of fibroblast movement happens amid the tissue renovating phase of wound healing. TGF-β1 has arranged parts in cutaneous injury mending, including a stimulatory action on re-epithelialisation, wound contracture, angiogenesis, scar development and ECM accumulation [26]. In particular, TGF-β1 rouses increased cell migration in dermal fibroblasts [27] and keratinocytes [28]. Investigations of knock-out mice have suggested that IL-6 structures a key component of the standard cutaneous injury recuperating process by controlling dermal fibroblast relocation [29], has likewise been indicated to invigorate keratinocyte [30] and proliferation [31,32].
Conclusion
We have demonstrated the process of angiogenesis, and healing of burn wounds, using stromal cells. Healing of wounds remains an integral function of modern surgical science, for all surgical patients and especially those with burn injuries. The biology of wound healing entails many integrated, parallel processes that lead to the decontamination and closure of a wound. Angiogenesis is a fundamental process for wound mending/healing. It is also involved in the pathogenesis of a few ailments. Our study gives a hint about the use of SVFs in treating injuries.
References
- Harding K, Morris H, Patel G. Science, medicine, and the future: healing chronic wounds. Br Med J 2002; 324:160-163.
- Metcalfe AD, Ferguson MW. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J Royal Society Interface 2007; 4:413-437.
- Telci D, Ilter AZ, Erdem M. Stem Cells in Wound Healing Stem Cells: Current Challenges and New Directions. Springer, Berlin, 2013.
- Telci D, Griffin M. Tissue transglutaminase (TG2)-a wound response enzyme. Frontiers in bioscience: a journal and virtual library 2005; 11:867-882.
- Ko SH, Nauta A, Wong V. The role of stem cells in cutaneous wound healing: what do we really know? PlastReconstSurg 2011; 127:10S-20S.
- Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 2011; 6: 457-478.
- Gimble JM. Adipose tissue-derived therapeutics. Expert OpinBiolTher 2003; 3: 705-713.
- Lau K, Paus R, Tiede S, Day P, Bayat A. Exploring the role of stem cells in cutaneous wound healing. ExpDermatol 2009; 18: 921-933.
- Wolf NS. Cell Replication Rates In Vivo and In Vitro and Wound Healing as Affected by Animal Age, Diet, and Species The Comparative Biology of Aging. Springer, Berlin, 2010, 97-122.
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7: 211-228.
- Auer JA, Goodship A, Arnoczky S, Pearce S, Price J. Refining animal models in fracture research: seeking consensus in optimising both animal welfare and scientific validity for appropriate biomedical use. BMC MusculoskelDisord 2007, 8:72-85.
- Damy SB, Camargo RS, Chammas R, Figueiredo LF. Fundamental aspects on animal research as applied to experimental surgery. Rev Assoc Med Bras 2010; 56: 103-111.
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI. Human adipose tissue is a source of multipotent stem cells. MolBiol Cell 2002; 13: 4279-4295.
- Rodriguez AM, Elabd C, Amri EZ, Ailhaud G, Dani C. The human adipose tissue is a source of multipotent stem cells. Biochimie 2005; 87: 125-128.
- Li Y, Fan J, Chen M. Transforming growth factor-alpha: a major human serum factor that promotes human keratinocyte migration. Journal of investigative dermatology 2006; 126:2096-105.
- Faustini M, Bucco M, Chlapanidas T. Nonexpandedmesenchymal stem cells for regenerative medicine: yield in stromal vascular fraction from adipose tissues. Tissue Eng C 2010; 16:1515-1521.
- Gilpin DA. Calculation of a new Meeh constant and experimental determination of burn size. Burns 1996; 22:607-611.
- Kaplan SA. Expression of Vascular Endothelial Growth Factor Receptor-3 (VEGFR-3) in Human Prostate. J Urol 2004; 172:1578.
- Li R, Younes M, Wheeler TM, Scardino P, Ohori M. Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) in human prostate. Prostate 2004; 58: 193-199.
- Basaran Ö, Özdemir H, Kut A. Effects of different preservation solutions on skin graft epidermal cell viability and graft performance in a rat model. Burns 2006; 32:423-429.
- Ge L, Sun L, Chen J, Mao X, Kong Y. The viability change of pigskin in vitro. Burns 2010; 36: 533-538.
- Smith AN, Willis E, Chan VT, Muffley LA, Isik FF. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res 2010; 316: 48-54.
- Townsend Jr CM, Beauchamp RD, Evers BM.Sabiston Textbook of Surgery: Expert Consult Premium Edition: Enhanced Online Features. Health Sci 2012.
- Kumar V. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, Pa.: Saunders Elsevier; 2010.
- Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci 2013; 70: 2059-2081.
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008; 16: 585-601.
- Postlethwaite A, Keski-Oja J, Moses H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J ExpMed 1987; 165:251-256.
- Li AI, Hokugo A, Jarrahy R. Human Adipose Tissue as a Source of Multipotent Stem Cells. Stem Cell AesthetProced 2014.
- Luckett LR, Gallucci RM. Interleukin-6 (IL-6) modulates migration and matrix metalloproteinase function in dermal fibroblasts from IL-6KO mice. Br J Dermatol 2007; 156: 1163-1171.
- Gallucci RM, Sloan DK, Heck JM, Murray AR, O'Dell SJ. Interleukin 6 indirectly induces keratinocyte migration. J Invest Dermatol 2004; 122: 764-772.
- Sato M, Sawamura D, Ina S. In vivo introduction of the interleukin 6 gene into human keratinocytes: induction of epidermal proliferation by the fully spliced form of interleukin 6, but not by the alternatively spliced form. Arc DermatolRes 1999; 291:400-404.
- Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J 2000; 14: 2525-2531.