ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2011) Volume 22, Issue 1
Role of Tumor necrosis factor alpha, Malondialdehyde & serum Iron in Anemic Tuberculosis Patients
Rashmi kulkarni1*, Ajit Deshpande2, Kiran Saxena3, Akhouri RS Sinha4, Meena Verma5, Ravi Saxena6
1Department of Biochemistry Shri Aurobindo Institute of Medical Sciences, India
2Department of Community Medicine Shri Aurobindo Institute of Medical Sciences, India
3Department of Biochemistry Chirayu Medical College Hospital, Bhopal, India
4Department of Biochemistry College of Medical sciences, Amargadh. India
5Department of Biochemistry MGM Medical College, Indore, India
6Department of Physiology. College of medical sciences, Amargadh, India
- Corresponding Author:
- Rashmi A Kulkarni
Department of Biochemistry
Shri Aurobindo Institute of Medical Sciences
Indore, Madhya Pradesh
India
Accepted Date: September 19 2010
The role of Tumor Necrosis Factor alpha, Malondialdehyde and serum Iron in inducing anemia in Tuberculosis patients was studied. The present study was conducted in the De-partment of Biochemistry, Shri Aurobindo Institute of Medical Sciences, Indore. The study comprised of sixty-eight anemic pulmonary tuberculosis patients. Levels of Hemoglobin, Iron, TIBC, Ferritin, MDA & TNF α were analyzed in all of them. The inflammatory pa-rameters TNF α & Ferritin along with oxidative marker malondialdehyde were found to be increased in anemic tuberculosis patient and levels of Hb, iron & TIBC was decreased. Overall correlation shows that the levels of Hb, Iron & TIBC were negatively correlated to the levels of Ferritin, MDA & TNF α. Ferritin was negatively correlated with Iron (r = -0.261, p<0.05) & showed a positive correlation with MDA. (r = 0.304, p < 0.05). For Ferritin & TNF α correlation was positive, it was not significant (r = 0.114, NS).There was a positive significant correlation between Iron & TIBC. (r = 0.666, p<0.01) & a negative significant correlation between the TNF α levels and the hemoglobin concentration (r = -.273, p<0.05). Hypoferremia was induced as a consequence of inflammatory response and action of free radicals in anemic tuberculosis patients
Keywords
Anemia, Hypoferremia, Ferritin, TNF α , Malondialdehyde.
Introduction
Active tuberculosis infection is generally associated with major abnormalities in heamopoiesis [1]. Anemia is a common complication of pulmonary tuberculosis, the reported prevalence ranging from 16 to 76% in different studies [2]. The precise mechanism of anemia in pulmo-nary tuberculosis patients (PTB) is not clearly known however, anemias due to inflammation & iron deficiency have been implicated [3,4]. The pathogenesis of anemia of chronic inflammation may evolve due to cytokine-mediated defense against microbial pathogens, effectively withholding iron from microbes, which incidentally also deprive erythroid precursors of their iron supply [5,6,7]. The development of hypoferremia thus results in the de-velopment of iron-restricted erythropoiesis and anemia. Cytokine also induced alterations in cellular iron homeo-stasis are mediated in part by the increased production of nitric oxide (NO) and reactive oxygen species (ROS) [8].
The present study was taken to find out the role of Tumor Necrosis Factor alpha, Malondialdehyde & serum Iron in inducing Anemia in Tuberculosis Patients.
Material and Method
Present study was conducted in the Department of Bio-chemistry, Shri Aurobindo Institute of Medical sciences, Indore. Sixty-eight patients of anemic pulmonary tubercu-losis were selected. The patients of pulmonary tuberculo-sis were taken from Shri Aurobindo Institute of Medical Sciences and Manorama Raje Tuberculosis Hospital, In-dore. Pulmonary tuberculosis patient falling in DOTS category CAT I & aged between 18-55 yrs & hemoglobin (Hb) less than <13 g/dL for Men & HB <12 g/dL for non-pregnant Women was included. Tuberculosis patient with other disease or disorder and patients with incomplete data records and moderate to severe injury or surgery dur-ing the previous month were excluded. Those patients who have taken antibiotics for cough like Amoxicillin, Septran, Ciprofloxacilln etc were included but those pa-tients with history of prior anti-TB treatment were not included. Patient with extra pulmonary involvement, blood donors, subjects with history of smoking and who had taken iron supplementation were excluded. The study was approved by the Institutional & Human Ethical Committee of the Sri Aurobindo Institute of Medical sci-ences, Indore. Informed consent was taken from each sub-ject and then they were enrolled in the study.
5ml of fasting venous blood samples were drawn from subjects, each sample was divided into two parts: the first part was collected in EDTA while the second sample was collected in a plain tube and left to clot. Serum was ob-tained after centrifuge. The serum samples were aliquoted and stored according to the instructions in the kits, until the various assays described below were performed
Haemogram was estimated by automated cell counter. (Sysmex KX) Serum iron and total iron-binding capacity (TIBC) were estimated on an autoanalyser from Rosh diagnostics using commercial test kits from Randox La-boratories, UK. Ferritin was estimated by direct immu-noenzymatic determination by using test kits from Dia-metra, Italy. Serum TNF α was estimated by using a commercially available immunoassay Kit from DIA-CLONE,France. Serum Malondialdehyde was estimated spectrophotometrically by thiobarbituric acid method of Wilbur K M et al (1949) [9].
Correlations were calculated by Pearson’s correlation co-efficients (two-tailed) using SPSS Version 10. P value ≤ 0.05 was used as a threshold of significance.
Result
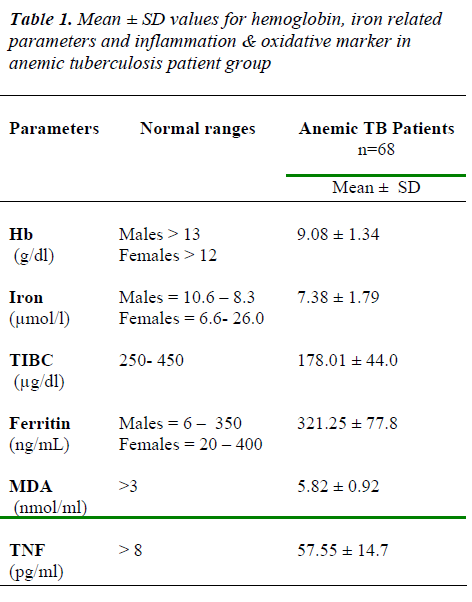
The levels of Hb, iron & TIBC was decrease than normal ranges & ferritin, MDA & TNF values found to be in creased than normal ranges in anemic tuberculosis pa-tients (Table 1)
Pearson correlations (Table no 2) were carried out on the different variables, which showed that the decreasing lev-els Hb, Iron & TIBC was negatively correlated to the in-creasing levels Ferritin, MDA & TNF α.
The statistically significant correlation was found be-tween Hb & TNF α (r = -0.273, p<0.05), Iron & TIBC ( r = 0.666, p<0.01) , Iron & ferritin (r = -0.261, p< 0.05) & Ferritin & MDA (r = 0.304, p < 0.05).
Discussion
Anemia is one of the abnormalities found in TB Patients due to poor nutritional causes and increased in cytokines level 1. Therefore, both anemia of inflammation as well as iron deficiency anemia may coexist in pulmonary tuber-culosis. Our findings were consistent with the previous reports that there was decrease in Hb, Iron & TIBC levels and increased levels of ferrtin, TNF α & MDA in tubercu-losis patients [10,11,12,13,14,15].
Hypoferremia in disease associated anemia appears to be the result of a complex network of cytokines, immune cells, and acute-phase proteins [16]. TIBC values are usu-ally high in iron-deficiency anemia and low in anemia of inflammation [17,18]. The low TIBC concentrations seen in our present patients might have been the result of an acute-phase response. Ferritin concentration in blood is considered a specific indicator of body iron stores [19], however, the concentrations can rise following an in-flammatory response, irrespective of iron status [20,21,23,].
Endogenous TNF α response during inflammation is a sufficient proximal stimulus to mediate an associated anemia with decreased RBC survival & impaired erythro-poiesis [24].
Recombinant TNF α and recombinant interleukin 1 (IL-1) cause a significant decrease in serum iron levels accom-panied by an increase in circulating ferritin concentration and a decrease in soluble transferrin receptor levels [25,26]. Increased levels of TNF α , ferritin & decreased iron levels in present study & their correlation with each other was in accordance with the finding that tumour ne-crosis factor alpha induce hypoferraemia and ferritin pro-duction [16]. Similar findings was stated by Bayes et al [11] for C-reactive protein (CRP), iron & ferritin.
The increased levels of TNF α & MDA & decreased lev-els of Hb, Iron, TIBC reveals that the MDA along with TNF α was involve in redistribution of iron. These find-ings are in concordance with the literature [26]. Cytokines affect iron homeostasis in monocytes by inducing the formation of short-lived radicals such as nitric oxide (NO) and reactive oxygen species (ROS) which affect the post-transcriptional regulation of iron homeostasis via iron regulatory proteins (IRPs) [27,28].
In anemic tuberculosis patients disturbances in iron ho-meostasis may be one of the cause to the shift of iron from a transferrin- bound available state to a ferritin-incorporated storage state. These changes are the compo-nents of a systemic inflammatory response and action of free radical, which may be one of the major factors in the pathogenesis of anemia.
References
- Sei Won Lee, Young Ae Kang, Young Soon Yoon, et al. The Prevalence and Evolution of Anemia Associ-ated with Tuberculosis. J Korean Med Sci 2006; 21: 1028-32.
- Goldenberg AS. Haematological abnormalities and mycobacterial infections. In Tuberculosis 1996; pp. 645–652 [WN Rome and S Garay, editors]. Boston, MA: Little Brownand Company.
- Douglas SW & Adamson JW. The anaemia of chronic disorders: Studies of marrow regulation and iron me-tabolism. Blood 1975; 45: 55–65
- Das BS, Nanda NK, Rath PK, et al. Anaemia in acute, Plasmodium falciparum malaria in children from Orissa state, India. Ann Trop Med Parasitol 1999;93: 109–118.
- Bullen JJ, Rogers HJ & Griffiths E. Role of iron in bac-terial infection. Curr Top Microbiol Immunol 1978; 80: 1-35.
- Weinberg E. Iron and infection. Microbiol Rev 1978 ; 42: 45-66.
- Jurado RI. Iron, infection, and anaemia of inflamma-tion. Clin Infect Dis 1997; 25: 888-895
- Igor Theurl, Verena Mattle, Markus Seifert, et al. Dys-regulated monocyte iron homeostasis and erythropoi-etin formation in patients with anemia of chronic dis-ease. Blood may 2006; 107(10): 41 4142-4148
- Wilbur KM, Bernheim F, Shapiro OW. The thiobarbi-turic acid reagent as a test for the oxidation of unsatu-rated fatty acid by various agents. Arch Biochem Bio-physic 1949;24:305-313
- Uma Devi, C. Mohan Rao, Vinod K. Srivastava, et al. Effect of iron supplementation on mild to moderate anaemia in pulmonary tuberculosis. British Journal of Nutrition 2003; 90: 541-550
- Baynes RD, Flax H, Bothwell TH, et al. Hematological and iron-related measurements in active pulmonary tu-berculosis. Scand J Haematol 1986; 36: 280-7
- Kaminskaia GO, Abdullaev RIu. Iron metabolism in patients with different variants of pulmonary tuberculo-sis Probl Tuberk. 2002; 12: 49-51
- Kaminskaia GO, Abdullaev RIu, Baturova GA, et al. The specific features of iron intake in patients treated for pulmonary tuberculosis Probl Tuberk Bolezn Legk. 2009; 7: 46-55
- Nakaya M, Yoneda T, Yoshikawa M, et al. The evalua-tion of interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-alpha) level in peripheral blood of patients with active pulmonary tuberculosis. Kekkaku. 1995; 70 8: 461-466.
- Kharakter ZhZ, Skoraia RI, Platonova IL. The ceru-loplasmin and lipid peroxidation indices in patients with pulmonary tuberculosis. Vrach Delo.1989; 10: 55-7
- Feelders RA, Vreugdenhil G, Eggermont AM, et al. Regulation of iron metabolism in the acute-phase re-sponse: interferon gamma and tumour necrosis factor alpha induce hypoferraemia, ferritin production and a decrease in circulating transferring receptors in cancer patients. Eur J Clin Invest. 1998; 28: 520-527
- Fleck A & Myers MA. Diagnostic and prognostic sig-nificance of acute phase proteins. In The Acute Phase Response to Injury and Infection1 985:249–271 [AH Gordon and A Koj, editors]. Amsterdam: Elsevier Sci-ence Publishers.
- Punnonen K, Irjala K, Rajamaki A. Iron deficiency anaemia is associated with high concentrations of trans-ferring receptor in serum. Clin Chem 1994; 40: 774 - 776
- Lipschitz DA, Cook JD, Finch CA. A clinical evalua-tion of serum ferritin as an index of iron status. New Eng J Med 1974; 290: 1213-1216.
- Dallman PR, Reeves JD, Driggers DA, et al. Diagnosis of iron deficiency: the limitations of laboratory tests in predicting response to iron treatment in 1-year-old in-fants. J Pediatr 1981;98: 376- 381.
- Harju E, Pakarinen A & Larmi T. A comparison be-tween serum ferritin concentration and the amount of bone marrow stainable iron. Scand J Clin Lab Invest 1984; 44: 555-556.
- Henderson A Ferritin levels in patients with microcytic anaemia complicating pulmonary tuberculosis. Tuber-cle 1984;65 : 185-189.
- Taylor PG, Martinez-Torres C ,Mendez-Castellano H, et al. The relationship between iron deficiency and anaemia in Venezuelan children. Am J Clin Nutr 1993; 58: 215-218.
- Lyle L, Moldawer, Michael A. Marano, He Wei, Yu-man Fong, et al. Cachectin/tumor necrosis factor-a al-ters red blood cell kinetics and induces anemia in vivo. The FASEB Journal March 1989;. 3: 1637- 1643
- Alvarez-Hernandez X, Liceaga J, McKay IC, et al. In-duction of hypoferremia and modulation of macro-phage iron metabolism by tumor necrosis factor. Lab Invest. 1989; 61: 319-322
- Maciejewski, JP, Selleri C, Sato T et al. Nitric oxide suppression of human hematopoiesis in vitro. Contribu-tion toinhibitory action of interferon-gamma and tumor necrosis factor-alpha. J Clin Invest 1995; 96: 1085-1092
- Drapier JC, Hirling H, Wietzerbin J, et al. Biosynthesis of nitric oxide activates iron regulatory factor in ma-crophages. EMBO J 1993; 12: 3643-3649
- Cairo G, Recalcati S, Pietrangelo A, et al.The iron reg-ulatory proteins: targets and modulators of free radical reactions and oxidative damage.Free Radic Biol Med. 2002; 32: 1237-1243