ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2013) Volume 24, Issue 1
Satellite glial cells in the nodose ganglion of the rat vagus nerve: Morphological alterations of microglial cells.
1Department of Anatomy and Neurobiology, Faculty of Medicine, Kagawa University, 1750-1 Ikenobe, Miki-cho, Kagawa 761-0793, Japan
2Laboratory of Animal Science, Faculty of Agriculture, Kagawa University, 2393 Ikenobe, Miki-cho, Kagawa 761-0795, Japan
- *Corresponding Author:
- Yoshiki Takeuch
Department of Anatomy and Neurobiology
Faculty of Medicine, Kagawa University
1750-1 Ikenobe, Miki-cho
Kagawa 761-0793, Japan
Accepted Date: September 17 2012
Satellite glial cells (SGCs) have been well known to be present in the sensory and autonomic ganglia of the peripheral nerves and play an important role in various brain functions. However, interactions between the ganglion cells and SGCs involved in transcytosis of materials remained as a poorly-solved problem. In the present study, the cellular processes and molecular mechanisms of the SGCs were investigated in the nodose ganglion (NG) of the rat vagus nerve. Immunofluorescent experiments of the SGCs led to expression of GFAP- and Iba-1-immunoreactivity in close vicinity to neuronal cell bodies. However, very weak Iba-1 expression was seen in GFAP- immunoreactive cells. It was of particular interest that Iba-1- immunoreactive cells seem to be extending a part of the glial cell bodies into neuronal cell bodies in the NG. In electron microscopic experiments, injection of wheat germ agglutinin conjugated horseradish peroxidase (WGA-HRP) into the vagus nerve resulted in deep extension of the fine processes of the SGCs into neuronal cell bodies and uptake of HRPreaction product. These findings would indicate that the SGCs respond to environmental changes of neuronal cell bodies are reactive microglial cells in the NG.
Keywords
Satellite glial cells, Microglial cells, Fine processes, Iba-1, GFAP, Nodose ganglion.
Introduction
The peripheral nervous system includes specialized glial cells termed “satellite glial cells (SGCs)” in various ganglia [1,2,3]. The SGCs are known to be one type of glial cells surrounding neuronal cell bodies in sensory ganglia [2,4] and show a similar embryological origin to Schwann cells which are derived from the neural crest during development [5]. Interestingly, they are thought to have a similar role to microglia which shows equipped mobility functioning as the macrophages on the central nervous system [6]. In recent studies, the SGCs were also reported to have functions to supply nutrients to the surrounding neurons [7,8]. In addition, they expressed a variety of receptors that allow for a range of interactions with neuroactive chemicals [9]. It appears that the SGCs carry receptors for numerous neuroactive agents and can receive signals from other cells and respond to changes in their environment. Activation of the SGCs might in turn influence neighboring neurons. Thus, the SGCs are likely to participate in signal processing and transmission in sensory ganglia [9]. Morphological analysis onto the functional implication of the SGCs is considered to be useful for understanding the normal and pathological brain conditions.
Therefore, the present study was done to identify the reactive glial cells and elucidate the morphological alterations of the SGCs, particularly of their processes, in response to environmental changes of neuronal cell bodies of the nodose ganglion (NG) which were occurred by injection of horseradish peroxidase (HRP) into the rat vagus nerve.
Materials and Methods
Experiments were performed on male Wistar rats (SLC, Hamamatsu, Japan), weighing 280-340g. The animals were housed in separate cages and maintained under standard laboratory conditions (23 ± 1 oC, 12-h light: 12-h dark cycle, food and water ad libitum). The experimental procedures were conducted in accordance with National Institute of Health (NIH) for Care and Use of Laboratory Animals. The Kagawa University Animal Care and Use Committee approved the procedures (authorization number of the ethical approval: No. 78), and all efforts were made to minimize the number of animals used and their suffering.
Animals were anesthetized with intraperitoneal injection of chloral hydrate (490 mg/kg) for all surgical procedures. In the light microscopic experiment, a solution of Alexa Fluor 594 conjugate of WGA (Invitrogen, USA) were injected into the right vagus nerve using a 10 μl Hamilton microsyringe under the operation microscope (4 rats). The injection was made into the proximal end of the vagus nerve which was exposed widely around the common carotid artery at the neck portion. In this experiment the injected solution was clearly restricted along the course of the vagus nerve. Furthermore, to prevent leakage of injected solution, the proximal end of the vagus nerve was pinched with forceps for a few minutes. After a survival period of 2-3 days, the animals were perfused transcardially with 0.02M phosphate buffered saline (PBS, pH 7.4) followed by fixation with 4% paraformaldehyde and 0.1% glutaraldehyde in 0.02M PBS. For tissue cryosection analysis, the NG was processed with gradient infiltration up to 30% sucrose in 0.02M PBS and embedded in an optimal cutting temperature compound (Tissue -Tek). Serial 20 μm-thick frozen sections were processed for fluorescent immunohistochemical staining for GFAP and Iba-1 as following brief description. The sections were placed in 1% normal donkey serum (Jackson immunoresearch lab. West Grove, PA, USA) for 1 h and incubated with primary antibodies at 4oC overnight. PBS solution containing the primary antibodies included a mouse monoclonal anti-GFAP (BD Biosciences, USA) and a rabbit polyclonal anti-Iba-1 (Wako, Japan). Sections then were incubated with Alexa 594 conjugated anti-mouse IgG (Invitrogen, USA) and Alexa 488 conjugated antirabbit IgG (Invitrogen, USA) in PBS for 2 h. All treated slides were processed for nuclear counter staining with 50 ng/ml Hoechst 33258 (Sigma-Aldrich, USA) for 10 min, and mounted in fluorescence mounting medium (Vector lab, USA).
The slides were coverslipped using mounting medium (Vector lab). Immunofluorescent control studies were performed by omission of the primary antibodies. No respective labeling was detected under these conditions. Sections were analyzed by epi-illumination fluorescence microscope (DP-72, Olympus, Japan) and processed by an image analyzer (NIS-Elements D, Nikon, Japan) [10]. Confocal laser scanning microscope images were visualized on Radiance 2100 (Bio-Red, Hercules, CA) and an optical slice thickness of 0.2μm.
In the electron microscopic experiment, a solution of 4% WGA-HRP (Vector Laboratries, Inc) was also injected into the right vagus nerve at the neck portion according to the method of injection of fluorescent substance described above (5 rats). After a survival period of two days, animals were perfused with 0.1M phosphate buffer (pH7.4) followed by a fixative of 1% paraformaldehyde and 1.25% glutaraldehyde in 0.1M phosphate buffer. The blocks containing the NG were cut transversely into 200 μm-thick sections using a vibratome (Leica VT 1000S, Germany) and sections were processed for demonstration of HRP-RP according to the heavy metal-intensified diaminobenzidine method [11,12,13]. The sections were postfixed in buffered 1% osmium tetroxide for 2 h, blockstained in saturated uranyl acetate for 1 h, dehydrated in a graded alcohol series and embedded in an epoxy resin mixture as reported previously [14,15]. The NG was identified by examination of toluidine blue-stained or unstained 1 μm-thick sections. Ultrathin sections of the region were cut and observed without further lead staining using a JEOL 200 CX electron microscope.
As controls, the animals receiving no injection (3 rats) and the right vagotomy at the neck portion with a survival period of two days (5 rats) were used in the present study. These animals were perfused with the same fixative and sections containing the NG were postfixed, embedded in an epoxy resin mixture and observed according to the method of the electron microscopic experiment described above.
Results
Light microscopic observations
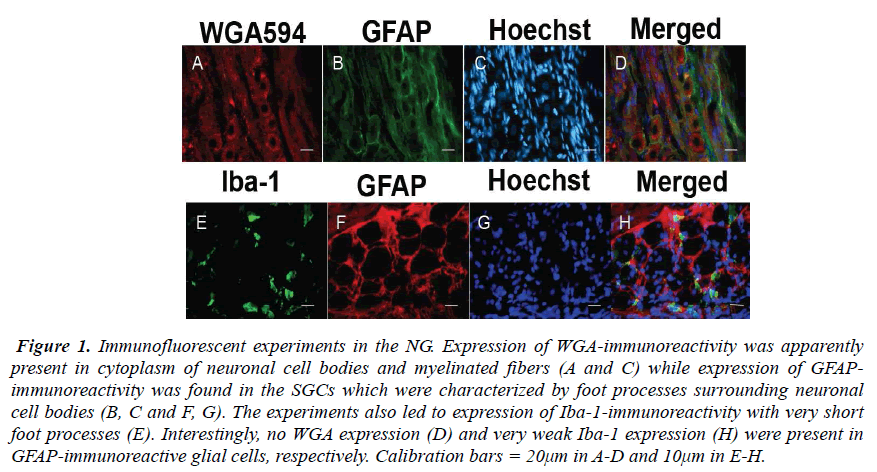
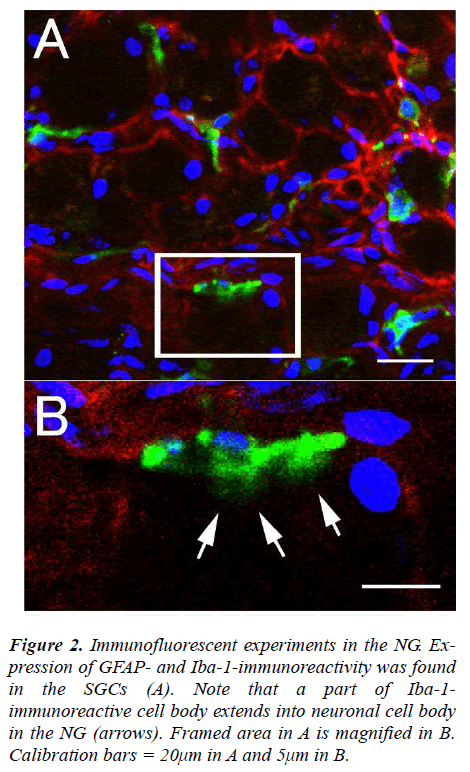
The NG of the vagus nerve was found at the rostral portion of the neck. The NG was slightly cylindrical in form and located just beneath the jugular foramen. After the ganglion was taken out from the vagus nerve, immunofluorescent staining was done. In the experiments, the SGCs share several properties with cells including WGA-, GFAP- and Iba-1-immunoreactivity in the NG. The expression of WGA-immunoreactivity was apparently observed in cytoplasm of neuronal cell bodies and myelinated fbers (Figs. 1A and C) while expression of GFAP-immunoreactivity was found in the SGCs characterized by foot processes surrounding neuronal cell bodies (Figs. 1B, C and F, G). Furthermore, the present experiments led to expression of Iba-1-immunoreactivity with short foot processes in close vicinity to the neuronal cell bodies (Fig. 1E). These Iba-1-immunoreactive cells were small in size and flattened or oval in shape (approximately 3.6×8.1μm). No WGA expression (Fig. 1D) and very weak Iba-1 expression (Fig. 1H) were observed in GFAP-immunoreactive cells, respectively. However, it was of particular interest in the present study that Iba-1- immunoreactive cells seem to be extending a part of their glial cells into neuronal cell bodies in the NG (Figs. 2A and B).
Figure 1: Immunofluorescent experiments in the NG. Expression of WGA-immunoreactivity was apparently present in cytoplasm of neuronal cell bodies and myelinated fibers (A and C) while expression of GFAPimmunoreactivity was found in the SGCs which were characterized by foot processes surrounding neuronal cell bodies (B, C and F, G). The experiments also led to expression of Iba-1-immunoreactivity with very short foot processes (E). Interestingly, no WGA expression (D) and very weak Iba-1 expression (H) were present in GFAP-immunoreactive glial cells, respectively. Calibration bars = 20μm in A-D and 10μm in E-H.
Figure 2: Immunofluorescent experiments in the NG. Expression of GFAP- and Iba-1-immunoreactivity was found in the SGCs (A). Note that a part of Iba-1- immunoreactive cell body extends into neuronal cell body in the NG (arrows). Framed area in A is magnified in B. Calibration bars = 20μm in A and 5μm in B.
Electron microscopic observations
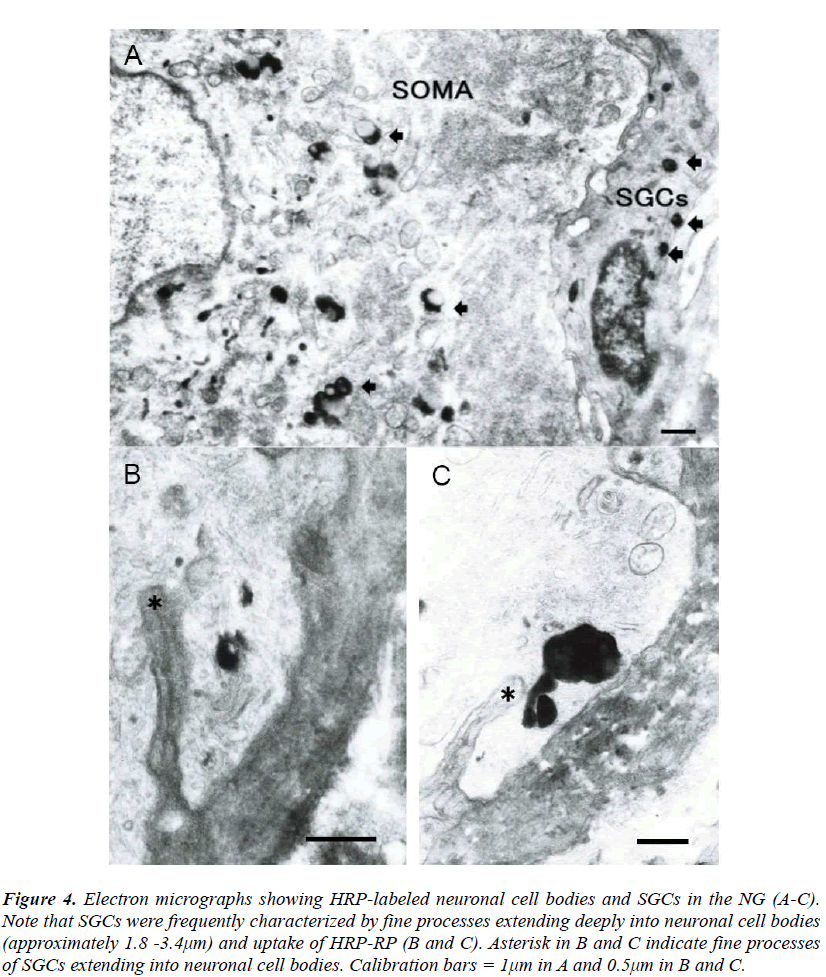
The SGCs were identified to be located in close vicinity to neuronal cell bodies in the NG. The SGCs were small in size and flattened in shape and had round or elliptical nucleus with centrally located nucleolus (Fig. 3). The NG was observed at the electron microscopic level after injection of WGA-HRP into the vagus nerve. HRP-reaction product (HRP-RP) was recognized as electron dense lysosomal- like substance when lead citrate staining was omitted. It was demonstrated that small sized cells having contacts with neuronal cell bodies are slightly labeled in the NG in contrast to heavy labeling of neurons (Fig. 4A). Interestingly, the small sized cells were characterized by some fine processes extending into neuronal cell bodies and uptake of HRP-RP (Figs. 4B and C). These cells were quite similar to the morphology of the SGCs identified as Iba-1-immunoreactive cells in immunofluorescent experiments. The depth of the fine processes extending into neuronal cell bodies was approximately 1.8-3.4μm. Materials phagocytosed by the fine processes finally seemed to be transported from neuronal cell bodies to the small sized cells in the NG (Figs. 4B and C). However, in the present study HRP-RP passing through membranes of neurons and small sized cells was not observed.
Figure 3: Photomicrograph of 1 μm-thick toluidine blue stained section of the NG in the intact animal. In the NG there are large sized neuronal cell bodies, small sized SGCs and myelinated fibers. Note that most of SGCs are situated in close vicinity to neuronal cell bodies of the NG (arrows). Calibration bar = 20μm.
Figure 4: Electron micrographs showing HRP-labeled neuronal cell bodies and SGCs in the NG (A-C). Note that SGCs were frequently characterized by fine processes extending deeply into neuronal cell bodies (approximately 1.8 -3.4μm) and uptake of HRP-RP (B and C). Asterisk in B and C indicate fine processes of SGCs extending into neuronal cell bodies. Calibration bars = 1μm in A and 0.5μm in B and C.
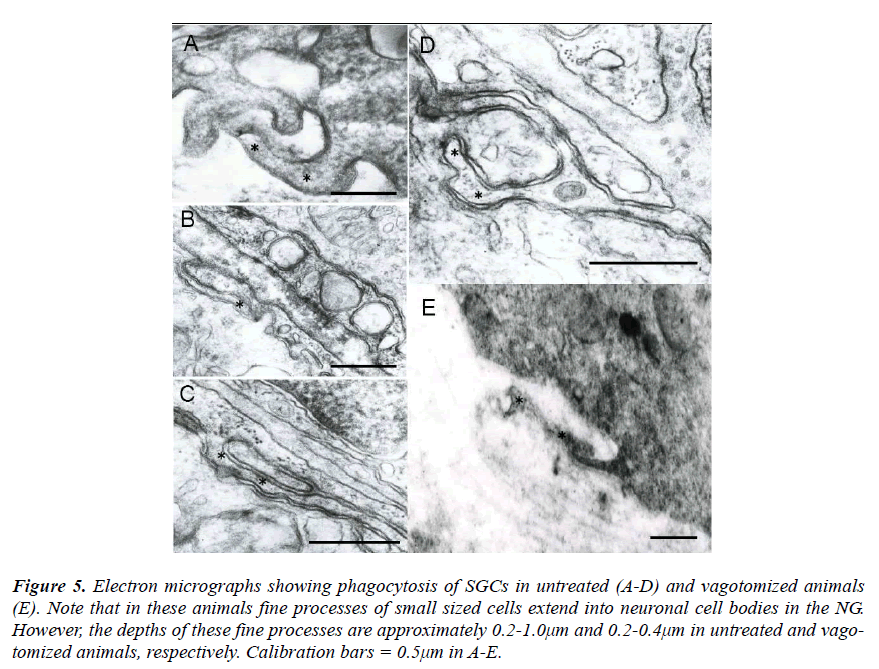
As control cases, the NG was investigated in untreated and vagotomized animals. In these animals the fine processes of small sized cells were also observed to extend into neuronal cell bodies (Figs. 5A-E). However, the depth of these fine processes was approximately 0.2- 1.0μm and 0.2-0.4μm in untreated and vagotomized animals, respectively.
Figure 5: Electron micrographs showing phagocytosis of SGCs in untreated (A-D) and vagotomized animals (E). Note that in these animals fine processes of small sized cells extend into neuronal cell bodies in the NG. However, the depths of these fine processes are approximately 0.2-1.0μm and 0.2-0.4μm in untreated and vagotomized animals, respectively. Calibration bars = 0.5μm in A-E.
Discussion
Neurons in sensory ganglia of cranial and spinal nerves have been well known to conduct impulses to synapses located in the central nervous system. However, it is of particular interest morphologically that the neurons are enveloped tightly by the SGCs [16]. This direct association with neurons might imply the SGCs providing some function of protective barrier [17]. These SGCs are considered to be neuroectoderm-derived and involved in the maintenance of homeostasis of sensory neurons by regulating extracellular ion and nutrient levels within sensory ganglia [4]. This means the possibility of having a distinct interaction with neurons.
In immunofluorescent experiments, the SGCs led to expression of GFAP- and Iba-1-immunoreactivity in close vicinity to neuronal cell bodies in the NG. However, the most conspicuous feature in the present study was the existence of a part of Iba-l immunoreactive cell bodies extending to neuronal cell bodies in the NG. This finding means to have a close relationship with microglial cells because Iba-1 is a marker for the microglial cells while GFAP is the major constituent of the intermediate filaments of the astroglial cells [18,19]. In addition, the characteristic structure of a part of Iba-1- immunoreactive cell bodies is considered to be identified as the fine processes of microglial cells at the electron microscopic level.
The fine processes of the SGCs were oserved to extend deeply into neuronal cell bodies and exert the uptake of HRP-RP after WGA-HRP injection was made into the vagus nerve. With respect to such phagocytic morphology of the SGCs, similar structure of the fine processes was found in the mouse dorsal root ganglion two weeks after sciatic nerve axotomy. However, the fine processes extended not into neuronal cell bodies but into connective tissue space [4]. Furthermore, degenerated neuroblastoma was observed to be completely surrounded and finally phagocytosed by SGCs in the spinal ganglia of chick embryo [20]. As a result, a phagocytic capacity was concluded to be probably a property common to SGCs of both sensory and autonomic ganglia of various species [20,21,22]. The appearance of the fine processes has a close relationship with environmental changes of neuronal cell bodies of the NG involving proteins and neuronal damages. In fact, in sensory neuronal damages the SGCs revealed morphological alterations and up-regulate growth factors [4, 23, 24]. Although in the present study effects of insertion of the needle on the SGCs should be considered, findings in the control cases might mean that damages by insertion of the needle into the vagus nerve exert weak effects on forming the fine processes when animals were perfused after a survival period of two days.
In conclusion, the immunofluorescent and HRP labeling methods identified the specific morphology of the SGCs in the NG as active microglial cells. The microglial cells were characterized by the fine processes extending deeply into neuronal cell bodies and exerting the uptake of HRPRP and finally transferring HRP-RP to the SGCs. However, slight extension of the fine processes into the neuronal cell bodies was also observed in untreated and vagotomized animals. It is possible that phagocytosis of the SGCs may be related to transport of materials including proteins from neuronal cell bodies to glial cells in order to have some important interactions such as protective and homeostatic functions. Further studies are required to test these possible hypotheses.
Acknowledgements
The authors acknowledge the skillful technical assistance of Mr. Wakashi Nagata and Mrs. Mizue Fukutomi. This investigation was supported by the grant for science research (No. 22591287) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- Pannese E. The structure of the perineuronal sheath of satellite glial cells (SGCs) in sensory ganglia. Neuron Glia Biol. 2010; 6: 3-10.
- Hanani M. Satellite glial cells in sympathetic and parasympathetic ganglia: in search of function. Brain Res Rev. 2010; 64: 304-327.
- Hanani M. Satellite glial cells: more than just 'rings around the neuron'. Neuron Glia Biol. 2010; 6: 1-2.
- Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Rev. 2005; 48: 457-76.
- Hall AK, Landis SC. Division and migration of satellite glia in the embryonic rat superior cervical ganglion. J Neurocytol. 1992; 21: 635-647.
- Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GK. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Rev. 1999; 30: 77-105.
- Hansson E, Rönnbäck L. Glial neuronal signaling in the central nervous system. FASEB J. 2003; 17: 341-8.
- Reichenbach A. Glial K+ permeability and CNS K+ clearance by diffusion and spatial buffering. Ann N Y Acad Sci. 1991; 633: 272-286.
- Shinder V, Devor M. Structural basis of neuron-toneuron cross-excitation in dorsal root ganglia. J Neurocytol. 1994; 23: 515-531.
- Fusumada K, Yokoyama T, Miki T, Matsumoto Y, Warita K, Wang ZY, Yakura T, Liu JQ, Takeuchi Y. GABA expression in c-Fos immunoreactive neurons of the rat periaqueductal gray induced by electroacupuncture at the point of Zusanli. Cur Neurobiol. 2010; 1: 10- 13.
- Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981; 29: 775.
- Liu JQ, Miki T, Warita K, Ohta K, Yakura T, Matsumoto Y, Takeuchi Y. Revaluation of neuronal contacts with pituicytes in the rat posterior pituitary. Cur Neurobiol. 2011; 2: 1-4.
- Takeuchi Y, Matsumoto Y, Miki T, Yokoyama T, Warita K, Wang ZY, Ueno T, Yakura T, and Fujita M. Anterograde synaptic transport of neuronal tracer enzyme (WGA-HRP): further studies with Rab3A-siRNA in the rat. Biomed Res. 2009; 20: 149-154.
- Takeuchi Y, Xie Q, Miki T, Matsumoto Y, Satriotomo I, Li HP, Gu H. Parabrachial inputs to Fos immunoreactive neurons in the lateral central nucleus of amygdale activated by hypotension: a light and electron microscopic study in the rat. Brain Res Bull. 2004; 64: 171- 180.
- Takeuchi Y, Matsumoto Y, Miki T, Warita K, Wang ZY, Bedi KS, Yakura T, Liu JQ. Alteration of the expression levels of Rab3A affects the extent of transcytosis of HRP-Labeled marker proteins in rat CNS neurons. Neurosci Med. 2011; 2: 282-287.
- Todd KJ, Serrano A, Lacaille JC, Robitaille R. Glial cells in synaptic plasticity. J Physiol Paris. 2006; 99: 75-83.
- Pannese E. The satellite cells of the sensory ganglia. Adv Anat Embryol Cell Biol. 1981; 65: 1-11.
- Takasaki C, Yamasaki M, Uchigashima M, Konno K, Yanagawa Y, Watanabe M. Cytochemical and cytological properties of perineuronal oligodendrocytes in the mouse cortex. Eur J Neurosci. 2010; 32: 1326-36.
- Patro N, Nagayach A, Patro IK. Iba1 expressing microglia in the dorsal root ganglia become activated following peripheral nerve injury in rats. Indian J Exp Biol. 2010; 48: 110-6.
- Pannese E. The response of the satellite and other nonneuronal cells to the degeneration of neuroblasts in chick embryo spinal ganglia. Cell Tissue Res. 1978; 190: 1-14.
- Matthews MR, Raisman G. A light and electron microscopic study of the cellular response to axonal injury in the superior cervical ganglion of the rat. Proc R Soc Lond B Biol Sci. 1972; 181: 43-79.
- Pilar G, Landmesser L. Ultrastructural differences during embryonic cell death in normal and peripherally deprived ciliary ganglia. J Cell Biol. 1976; 68: 339-56.
- Elson K, Simmons A, Speck P. Satellite cell proliferation in murine sensory ganglia in response to scarification of the skin. Glia. 2004; 45: 105-9.
- Lee SE, Shen H, Taglialatela G, Chung JM, Chung K. Expression of nerve growth factor in the dorsal root ganglion after peripheral nerve injury. Brain Res. 1998; 796: 99-106.