ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2006) Volume 17, Issue 2
Serotonergic neurons in the dorsal raphe nucleus are not activated by MK-801 during tooth movement
1Department of Developmental and Reconstructive Medicine, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan
2Department of Orthodontics and Craniofacial Developmental Biology, Hiroshima University Graduate School of Biomedical Sciences, Hiroshima, Japan
- *Corresponding Author:
- Tatsunori Shibazaki
Division of Orthodontics and Biomedical Engineering, Department of Developmental and Reconstructive Medicine
Nagasaki University Graduate School of Biomedical Sciences, 1-7-1 Sakamoto, Nagasaki 852-8588, Japan
Phone/Fax: 095-849-7669/7670
E-mail: tatsu-i ( at ) net.nagasaki-u.ac.jp
Accepted date: January 27, 2006
The dorsal raphe nucleus (DR), contains 60% serotonergic neurons, which appears to be involved in the control of endogenous pain sensation. MK-801, a non-competitive antagonist of N-methyl-D-aspartate receptors, inhibits spinal nociceptive neurons, however, little information is available about the effect of its administration on the DR. To evaluate the distribution of c-Fos and serotonin expression in the DR following experimental tooth movement in the rat upper molar and/or administration of MK-801. Fifty-six Wistar strain male rats underwent experimental tooth movement according to the Waldo’s method. Of all the rats, 28 were administrated MK-801 (3 mg/kg) intraperitoneally before initiating the experimental tooth movement (n=14). These animals were sacrificed 2 and 12 h after the experimental tooth movement. The sections including DR were dissected, and subjected to immunohistochemical staining with c-Fos and serotonin antibodies. In addition, 28 anesthesia control animals with or without administration of MK-801 were used as the controls. In the DR, the numbers of single-labeled (c-Fos) and double-labeled (c-Fos/serotonin) neurons were increased at 2 h and decreased by 12 h as compared with the controls. MK-801 decreased the numbers of double-labeled neurons at 2 h to the control level. The present results suggest that although blockage of NMDA receptors decreases neuronal activity with in the DR dur-ing experimental tooth movement, serotonergic DR neurons are not involved in the MK-801-induced analgesia
Keywords
c-Fos; Dorsal raphe nucleus; MK-801; experimental tooth movement
Introduction
It is well known that the dorsal raphe nucleus (DR) ap-pears to be involved in the control of endogenous pain sensation [1]. The DR contains many serotonin-like immunoreactive (5-HT-LI) neurons [2] and some of them project to the trigeminal sensory nucleus complex (TSNC) [3], which is a receptive field for orofacial nox-ious stimuli. Bullitt [4] reported that nucleus raphe mag-nus, which is involved in the modulation of nociception, underwent c-Fos expression following either noxious mechanical or noxious thermal stimulation. It is also demonstrated that c-Fos-like immunoreactive (Fos-LI) neurons are increased in the DR following experimental tooth movement [5]. However, it still remains unclear whether or not serotonergic DR neurons elicit c-Fos expression following experimental tooth movement.
It is reported that an intraperitoneal administration of MK- 801, a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist, exerts an antinociceptive effect on spinal nociceptive neurons in the rat following noxious stimulation [6]. Our previous study demonstrated that MK-801 increased c-Fos expression in the DR fol-lowing experimental tooth movement [7]. However, the type of excitatory amino acid (EAA) receptors involved in regulation of 5-HT neuronal discharge has not been clearly identified. Neurochemical evidence indicates that NMDA receptors may be one subtype involved in excita-tion of 5-HT neuronal activity [8]. Thus, NMDA elicits an increase in 5-HT release from primary cultures of fetal raphe neurons [9]. In order to clarify the action site of
NMDA in the DR, we investigated the effect of NMDA receptor antagonist on serotonergic DR neurons using immunohistochemical techniques following a noxious stimulation from experimental tooth movement. The aims of the present study were to evaluate, with a double labeling method, the induction of Fos-LI serotonergic neurons in the DR following experimental tooth movement and to examine the expression of serotonergic DR neurons in-duced by an intraperitoneal MK-801 administration.
Materials and Methods
Fifty-six Wistar strain male rats of 8-week-old, weighing 220-250 g, were used for this study. The experiment protocol was approved by the Committee for Animal Care and Use at Hiroshima University. For a series of experiments, the animals were divided into two experimental groups and three control groups. All rats were anesthetized intraperitoneally with sodium pentobarbital at a dose of 40 mg/kg b.w.
For fourteen rats in the first experimental group (TM group), a piece of orthodontic elastic module was inserted between the maxillary first and second molars on the right side under general anesthesia [10]. Two and 12 h after the insertion of the elastic module, the rats were sacrificed (n=7 for each time). In the second experimental group (TM/MK group), fourteen rats received an intraperitoneal injection of MK-801 (3 mg/kg b.w.) 30 min before the initiation of experimental tooth movement. They were also sacrificed 2 and 12 h (n=7, for each time) after initi-ating the experimental tooth movement.
In the first control group (normal control group), to exam-ine the basal expression of c-Fos, we sacrificed untreated animals immediately after the anesthesia (n=7). In the second control group (sham control group), seven rats underwent an insertion of elastic module between the right upper first and second molars, which was removed immediately after the insertion, and sacrificed 2 h later. In the third control group (MK control group), fourteen rats underwent the injection of MK-801 (3 mg/kg b.w.) 30 min before the insertion of elastic module. The elastic module was inserted between the first and second molars and removed immediately. They were sacrificed 2 and 12 h later (n=7, for each time).
For all the groups, the rats were deeply reanesthetized with an overdose of sodium pentobarbital, and perfused intracardially with 200 ml of normal saline, followed by a fixative of 500 ml containing 4% formaldehyde in 0.1 M sodium phosphate buffer (pH 7.2). The mid-brain was dissected, trimmed into blocks and immersed in the same fixative overnight, and then placed in 0.01 M phosphate-buffered saline (PBS) containing 25% sucrose at 4°C. The frozen blocks were cut into serial coronal sections of 50 μm thickness with a cryostat (Leica, Wetzlar, Germany) and stained with a double immunohistochemical staining for c-Fos and 5-HT. For the detection of c-Fos and 5-HT immunoreactivity, sheep polyclonal anti-c-Fos antibody and rabbit polyclonal anti-5-HT antibody were used as primary antibody, respectively. All the sections were rinsed in 0.01 M PBS, pre-incubated in absolute methanol plus 0.3% H2O2 to block endogenous peroxidase activity for 30 min, and then washed with PBS. The sections were incubated with sheep anti-c-fos antibody (Oncogene Research Products, Cambridge, USA) overnight at 4°C. Then, the sections were incubated sequentially with bioti-nylated donkey anti-sheep IgG (1:400; Vector, Burlin-game, USA) and then with avidin-biotin peroxidase complex (Dako, Glastrup, Denmark). The product of peroxidase activity was detected by using 0.04% 3,3’- diamino-benzidine, 0.1% ammonium nickel sulfate, and 0.01% hydrogen peroxidase in 0.05 M Tris-HCl buffer (pH 7.6). After washing in 0.01 M PBS, the sections were incubated with rabbit anti-5-HT antibody (Oncogene Research Products, Cambridge, USA) overnight at 4°C. Then, the sections were sequentially incubated with biotinylated goat anti-rabbit IgG (1:400; Vector, Burlingame, USA) and avidin-biotin peroxidase complex (Dako, Glastrup, Denmark). The product of peroxidase activity was detected by using Vector-VIP® in 0.01 M PBS. For each animal, the numbers of Fos-LI and Fos/5-HT-LI neurons were counted on both sides of the DR on four serial sections at an interval of 0.5 mm distance. These four levels range from 8.8 to 7.3 mm posterior to the Bregma. The stereotaxic coordinates in millimeters were taken from the atlas of Paxinos and Watson [11] using Bregma level. The average numbers of Fos-LI and Fos/5-HT-LI neurons per section were recorded for each Bregma level. ANOVA was used to determine whether statistically significant differences in the numbers of Fos-LI and Fos/5-HT-LI neurons existed between experimental and sham control groups. The differences in these values were examined with a Scheffé’s test.
Results
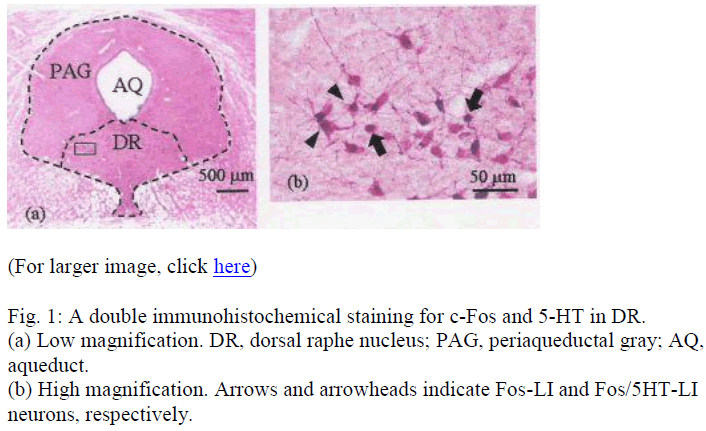
Under a light microscope (Olympus, Tokyo, Japan), Fos-LI and Fos/5-HT-LI neurons were observed in the DR. The cytoplasms of 5-HT-LI neurons were homogenously red (Fig. 1). Fos/5-HT-LI neurons were characterized by the presence of dark nucleus with homogenous red cyto-plasm (Fig. 1). Since the two reaction products are identi-fied clearly, two-type neurons with different labeling can easily be distinguished.
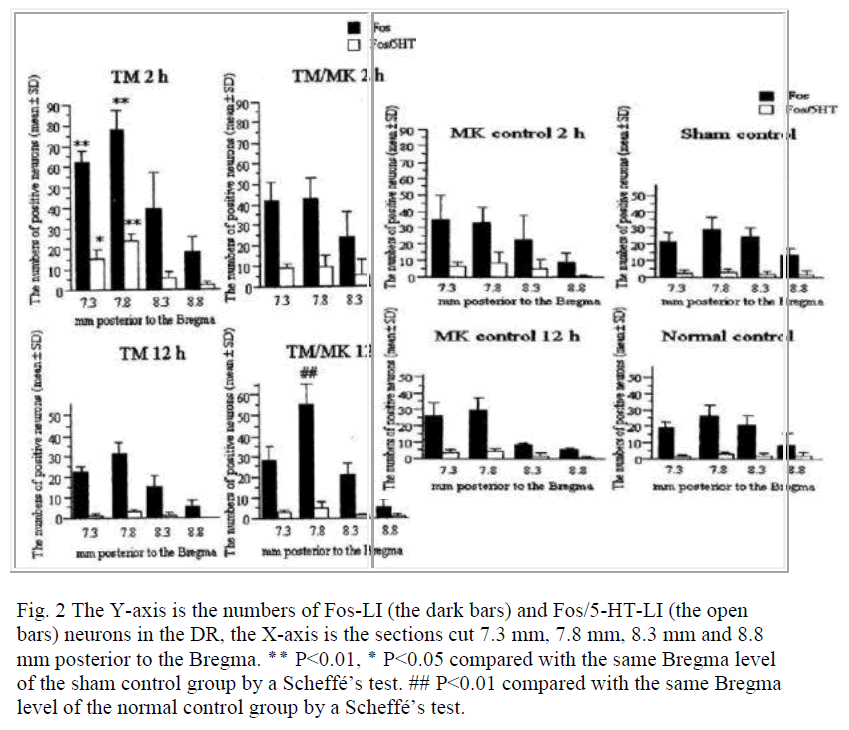
In the normal and sham control groups, a small number of Fos-LI and Fos/5-HT-LI neurons were detected in the DR for all the Bregma levels (Fig. 2). There were no significant differences in their numbers between the normal and sham control groups for all the Bregma levels.
Fig. 2 The Y-axis is the numbers of Fos-LI (the dark bars) and Fos/5-HT-LI (the open bars) neurons in the DR, the X-axis is the sections cut 7.3 mm, 7.8 mm, 8.3 mm and 8.8 mm posterior to the Bregma.
٭٭ P<0.01, ٭ P<0.05 compared with the same Bregma level
of the sham control group by a Scheffé’s test. ## P<0.01 compared with the same Bregma level of the normal control group by a Scheffé’s test.
In the TM group, the numbers of Fos-LI neurons and Fos/5-HT-LI neurons expressed at 2 h were 61.8±5.7 and 15.5±3.9 at the Bregma –7.3 mm, 77.4±9.1 and 24.0±3.1 at the Bregma –7.8 mm, 39.8±17.1 and 5.6±3.7 at the Bregma –8.3 mm, and 18.1±7.3 and 2.1±1.6 at the Bregma –8.8 mm, respectively. At the Bregma –7.3 and –7.8 mm, these numbers were significantly (P<0.05) larger than in the normal and sham control groups (18.5±3.9 and 25.9±6.9; 21.4±6.7 and 29.4±7.6; Fig. 2). At 12 h, no sig-nificant changes were found in the Fos-LI neurons as compared with those in the normal and sham control groups for all the Bregma levels.
In the TM/MK group, the numbers of Fos-LI neurons at 2 h at Bregma –7.3 and –7.8 mm (42.4±9.4 and 43.1±9.5) were not significantly greater than in the normal and sham control groups (Fig. 2). The numbers of Fos/5-HT-LI neu-rons at Bregma –7.3 and –7.8 mm (9.1±2.0 and 10.0±5.1) were not significantly different from those observed in the normal and sham control groups (Fig. 2). At Bregma –8.3 and –8.8 mm, the numbers of both neurons were almost similar to those in the sham control group. At 12 h, the numbers of Fos-LI neurons were significantly greater at Bregma –7.8 mm (54.9±10.5) than in the normal control group. The numbers of Fos/5-HT-LI neurons at all the Bregma levels were greater but not significant than those in the normal and sham control groups.
In the MK control group, the numbers of Fos-LI neurons at 2 h at Bregma –7.3 and –7.8 mm (34.9±14.5 and 33.3±9.8) were slightly but not significantly greater than those in the sham control group (Fig. 2). The numbers of Fos/5-HT-LI neurons at all the Bregma levels were not also significantly different compared with those observed in the sham control group (Fig. 2). At 12 h, the numbers of Fos-LI neurons at all the Bregma levels were decreased to the same level as the sham
Discussion
In the present study, the numbers of Fos-LI and Fos/5-HT-LI neurons in the DR increased 2 h after initiating ex-perimental tooth movement, although MK-801 admini-stration before experimental tooth movement induced no substantial increase in the number of Fos/5-HT-LI neu-rons in the DR. A previous study demonstrated that Fos-LI neurons were observed in the rat DR at 3 h following anterior tooth movement [5]. It is well known that the DR plays an important role in the control of endogenous pain sensation [1]. Therefore, DR neurons which appear to involve in the descending antinociceptive system ex-pressed c-Fos when they were activated appropriately. Formalin injection into a hindpaw [12] and unilateral electroacupuncture [13] also induced c-Fos in the DR. Moreover, noxious stimulation to the stomach induced Fos in the serotonergic DR neurons [14]. Our present finding, therefore, is in good agreement with above-mentioned results that various noxious stimulations in-duced c-Fos in the DR.
It is noteworthy that the DR receives a very dense gluta-mate input [15,16]. However, the type of relevant EAA-receptor involved has not been defined. Previous in vitro study of cultured raphe neurons indicates that NMDA can stimulate 5-HT release [9]. Activation of NMDA receptors in the nucleus is resulted from 5-HT release, and competitive and noncompetitive NMDA- receptor an-tagonists blocked the effect on extracellular serotonin in the DR [8]. MK-801 prevented an increase in serotonin release in the DR induced by electrical stimulation of medial prefrontal cortex projecting to the DR [17]. In agree-ment with these reports, serotonergic DR neurons were not activated by MK-801 during tooth movement in this study. Descarries et al. [18] demonstrated that approximately 40% of DR neurons are non-serotonergic in the rat. In addition to serotonin, the classic inhibitory neuro-transmitter GABA or GAD has been identified within a substantial number of DR neurons. Other putative trans-mitter substances including dopamine, NO, and a variety of neuropeptide have been found in cells within the borders of the DR. Although it is clear that DR neurons contain serotonin and neuroactive substances other than sero-tonin, there is little information about the neurochemical identity of DR neurons that project to functionally specify neuronal networks.
In conclusion, the present findings would provide new information that noxious stimulation to tooth activates serotonergic DR neurons and that MK-801 has no effects on these neuronal activities. Thus, NMDA receptors in the DR appear not to exert a function in regulation of 5-HT neuronal activity under the experimental conditions simulated in this study. It is thus speculated that MK-801 may modulate neuronal transmission in the TSNC directly without producing any neuronal activity in the DR.
Acknowledgment
This research was supported by a grant (No.16592045) for Science Research from the Ministry of Education, Science and Culture, Japan.
References
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spatial pathways and endorphin circuitry. Ann Rev Neurosci 1984; 7: 309-338.
- Li YQ, Zeng SL, Dong YZ, Rao ZR, Shi JW. Serotonin-, substance P- and tyrosine hydroxylase-like immunoreactive neurons projecting from the periaqueductal gray to the ventromedial hypothalamic nucleus in the rat. Neurosci Lett 1991; 134: 33-36.
- Senba E, Tohyama M, Shiosaka S, Takagi H, Sakanaka M, Matsuzaki T, Takahashi Y, Shimizu N. Experimental and morphological studies of the noradrenaline innervations in the nucleus tractus spinalis nervi trigemini of the rat with special reference to their fine structures. Brain Res 1981; 206: 39-50.
- Bullitt E. Expression of c-fos-like protein as a marker of neuronal activity following noxious stimulation in the rat. J Comp Neurol 1990; 296: 517-530.
- Magdalena CM, Navarro VP, Park DM, Stuani MB, Rocha MJ. c-fos expression in the rat brain nuclei fol-lowing incisor tooth movement. J Dent Res 2004; 83: 50-54.
- Haley JE, Sullivan AF, Dickenson AH. Evidence for spinal N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res 1990; 518: 218-226.
- Hattori Y, Watanabe M, Iwabe T, Tanaka E, Nishi M, Aoyama J, Satoda T, Uchida T, Tanne K. Administra-tion of MK-801 decreases c-Fos expression in the trigeminal sensory nuclear complex but increases it in the mid-brain during experimental movement of rat molars. Brain Res 2004; 1021: 183-191.
- Tao R, Auerbach SB. Differential effect of NMDA on extracellular serotonin in rat midbrain raphe and fore-brain sites. J Neurochem 1996; 66: 1067-1075.
- Becquet D, Faudon M, Hery F. In vivo evidence for an inhibitory glutamatergic control of serotonin release in the cat caudate nucleus: involvement of GABA neurons. Brain Res 1990; 519: 82-88.
- Waldo CM, Rothblatt JM. Histrogical responses to tooth movement in the laboratory rat. Procedure and preliminary observations. J Dent Res 1954; 33: 481- 486.
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Co-ordinates. Sydney: Academic Press 1998.
- Herdgen T, Zimmerman M. Immediate early genes (IEGs) encoding for inducible transcription factors (ITFs) and neuropeptides in the nervous system: func-tional network for long-term plasticity and pain. In f. Nyberg, H.S. Shama and Z. Wiessenbnfeld-Hallin (Eds.) Progress Brain Research vol. 1047. Amsterdam: Elsevier 1995; 299-321.
- Dai JL, Zhu YH, Li XY, Huang DK, Xu SF. C-fos expression during electroacupuncture analgesia in rats-an immunohistochemical study, Acupunct. Electrother Res 1992; 17: 165-176.
- Dong YX, Han ZA, Xiong KH, Rao ZR. Fos expression in serotonergic midbrain raphe dorsalis of adult rat: a light and electron microscope radioautographic study. Neurosci Res 1997; 27: 155-160.
- Kalen P, Strecker RE, Rosengren E, Bjorklund A. Reg-ulation of striatal serotonin release by the lateral habenula-dorsal raphe pathway in the rat as demonstrated by in vivo microdialysis: role of excitatory amino acids and GABA. Brain Res 1989; 492: 187-202.
- Nicholas AP, Peribone VA, Arvidsson U, Hokfelt T. Serotonin-, substance P and glutamate/aspartate-like immunoreactivities in medullo-spinal pathways of rat and primate. Neuroscience 1992; 48: 545-559.
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA, and glutamate receptors. J Neurosci 2001; 21: 9917-9929.
- Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radiographic study. J Comp Neurol 1982; 207: 239-254.