ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 2
Serum total superoxide dismutase enzyme activity in type 2 diabetic patients with retinopathy in Mthatha region of the Eastern Cape Province of South Africa
Division of Medical Biochemistry, Department of Human Biology, Faculty of Health Sciences, Walter Sisulu University, Nelson Mandela Drive, Mthatha-5100, South Africa
- *Corresponding Author:
- Ganjifrockwala FA
Department of Human Biology, Faculty of Health Sciences
Walter Sisulu University, South Africa
Accepted date: May 10, 2016
Superoxide dismutase is an important antioxidant enzyme and plays an important role against oxidative stress by catalyzing dismutation of superoxide radical. The aim of the present study was to evaluate total superoxide dismutase enzyme activity and total antioxidant levels in type 2 diabetic patients with retinopathy in the region and to compare it with that of type 2 diabetics without retinopathy and healthy non-diabetic controls. 140 participants were recruited with their consent and divided into three groups (44 with type 2 diabetics with retinopathy, 54 type 2 diabetics without retinopathy and 42 healthy nondiabetic controls). Fasting plasma glucose (FPG) and glycosylated hemoglobin were assayed by routine laboratory methods. Serum total superoxide dismutase (SOD) enzyme activity and total antioxidant (TAO) levels were measured using standard commercial reagent kits. The results are expressed as mean ± SD and median (IQR). There was statistically significant increase seen in FPG, HbA1c, and SOD enzyme activity in type 2 diabetic patients with and without retinopathy as compared to control population, whereas total antioxidant levels was significantly decreased in diabetic patients. Significant negative correlation was seen between TAO and SOD enzyme activity in diabetic group. SOD and duration of disease were found to be significantly associated with the presence of retinopathy in multiple logistic analyses. Present study strongly suggests the role of oxidative stress in the pathophysiology of diabetic retinopathy as is evident by the increased SOD activity and the decreased total antioxidant levels, thus making SOD a probable oxidative stress biomarker.
Keywords
Diabetes mellitus, Oxidative stress, Total antioxidant status, Superoxide dismutase.
Introduction
Diabetes mellitus is a group of metabolic disorders mainly characterized by hyperglycemia which occurs due to body’s inability to synthesize insulin or utilize insulin to its full potential [1,2]. It is a lifelong progressive metabolic disease affecting more than 230 million people worldwide and this number is expected to reach 350 million by year 2025.It is the fourth leading cause of death by disease globally and has become one of the most challenging health problem of 21st century [2,3]. In a study by Bertram et al. on the prevalence of type 2 diabetes in 2009, they reported a potential increase from 5.5% to 9% in people aged 30 or older since the previous estimates of year 2000 representing approximately 2 million cases of diabetes in South Africa. Their secondary studies also revealed that around 55% of cases are undiagnosed for South Africa meaning around 1 million people with type 2 diabetes are unaware of their disease. They also modelled 8000 new cases of blindness and 2000 new amputations annually caused by diabetes [4].
Diabetic retinopathy, one of the complication of diabetes mellitus is the fifth leading cause of blindness in the world, affecting 1.8 billion people and responsible for 4.8% of blindness. In South Africa, after cataract and glaucoma, retinopathy is the third leading reason of blindness [5]. Diabetic retinopathy is characterized by gradual and progressive alterations in vascular system of retina due to chronic hyperglycemia [6]. Diabetic retinopathy is classified into background or non-proliferative diabetic retinopathy (BDR/NPDR) and Proliferative diabetic retinopathy (PDR) [7-9]. The pathogenesis of diabetic retinopathy is very complicated and many factors contribute to it which include persistent hyperglycemia in retinal vasculature leading to build-up of advanced glycation end-products (AGEs), inflammation, neuronal dysfunction, changes in redox homeostasis and oxidative stress [2,6,10].
Reactive oxygen species production can be increased in numerous biochemical pathways as a result of hyperglycemia, which can have a potential to bring about adverse changes in endothelial function [11]. Superoxide dismutase’s (SOD) are major cellular defense against superoxide and peroxynitrite in a group of oxidoreductases and catalyzes the dismutation of superoxide into oxygen and hydrogenperoxide [12-17]. In mammals, it exists in three major isoforms characterized by the metals they contain and by their function to dismutate superoxide [18,19]. The three isoforms include SOD1 (cytosolic Cu-Zn-SOD), SOD2 (mitochondrial Mn-SOD) and extracellular SOD3 (EC-SOD) catalyze the same reaction but product of distinct gene and distinct sub cellular location [12,14-17,20,21]. SOD1 is a major intracellular SOD and is a dimer having molecular mass of 32 kDa [12,16,18]. It is widely distributed in cytosol of all mammalian cells and smaller fraction in the inner membrane space of mitochondria, nuclei, lysosomes and peroxisomes [16,18,22]. Presence of Cu and Zn is important for the enzymatic activity of SOD1 [16]. SOD2 (Mn-SOD) is a mitochondrial manganese (Mn) containing enzyme located in the mitochondrial matrix [12,16,22]. It is a tetramer with a molecular weight of 96kDa, synthesized in the cytosol and directed to the mitochondria by a signal peptide, where it dismutates superoxide produced by the respiratory chain. Mn at the active sites of SOD2 catalyzes the dismutation of superoxide to oxygen and water [12,16].
SOD3 (extracellular SOD) is a major SOD in the vascular extracellular space and is a homotetramer with molecular weight of 135 kDa [12,16,17]. Mainly, it is located in the extra cellular matrix (ECM) and on the cell surfaces with small fraction in the plasma and extracellular fluids. 90-99% of SOD3 in the body is tissue SOD and is different among species. Generally, it is expressed in selected tissues like blood vessels, lung, kidney, uterus and limited in heart. SOD3 is predominantly made by vascular smooth muscle cells and fibroblasts in vascular tissue and when secreted binds to the extracellular matrix such as heparin sulphates on the endothelial cell surface and is taken in by endothelial cells. All three SOD enzymes have various biological effects partly through H2O2, a product of superoxide dismutation, since H2O2 can act as a signaling molecule and activate many cellular responses like hypertrophy, proliferation, migration etc. [16]. Hyperglycemia in diabetes activates various biochemical pathways leading to increased production of superoxide and hydroxyl radical, which could lead to decrease in SOD enzyme activity. The present study was carried out to evaluate the total antioxidant levels and SOD enzyme in serum in diabetic patients and to check if there was any increase/ decrease in the levels of SOD enzyme in diabetic retinopathy patients.
Materials and Methods
The Higher Degrees Committee approved the study proposal and ethical clearance was obtained from Research Ethics Committee of the Faculty of Health Sciences, Walter Sisulu University (Bioethics clearance certificate No: 012/012)
Research participant recruitment
Inclusion criteria: Type 2 diabetic patients that belonged to indigenous African ethnicity and within age group (35-75 years) attending selected diabetic clinic (Gateway clinic) and ophthalmological clinic (Eye clinic, NMAH) in Mthatha, with and without retinopathies were approached and included in the study. Diabetic patients having hypertension and on insulin treatment were also included.
Exclusion criteria: Patients with HIV infection and other chronic diseases like tuberculosis, thyroid problems, heart problems, kidney failure if reported, were not included in this study. Patient taking multivitamins and statins were also excluded from the study. The clinical examination of the type 2 diabetic patients was carried out by a clinician of the diabetic clinic (Gateway clinic) and participants for the study were selected, keeping in mind the exclusion criteria.
They were then referred to the eye clinic of Nelson Mandela Academic Hospital (NMAH), where special arrangements were made for screening of these patients for the presence of retinopathies. All patients in the eye clinic were examined with dilated pupils through +90 lenses and indirect ophthalmoscope by an ophthalmologist. Fundus camera with high resolution was used for further confirmation and recording of fundus. Although diabetic retinopathy was classified through ETDRS (Early treatment diabetic retinopathy study), the various grading was not taken into consideration in this study and was broadly classified as background retinopathy and proliferative retinopathy due to the small sample size. Both type 2 diabetic patients with and without retinopathies were included in the study after their retinopathy status was confirmed and grouped accordingly. Control participants were selected by convenience sampling method from the general population keeping in mind the selection criteria.
Thus, total of 140 participants were included by convenient sampling method and subdivided into three groups as follows: 42 control healthy participants (without diabetes), 54 type 2 diabetics without retinopathy and 44 type 2 diabetic with retinopathy (28 with BDR and 16 with PDR). The participants were sensitized on the nature, importance of the study and explained that blood drawn from them for patient monitoring investigations will also be subjected to additional tests. The participants were asked to sign an informed consent form explaining purpose, procedures, risks and benefits of the study after their consent to volunteer for the study. A questionnaire form was used to obtain general information from the participant on medication, physical activity, family history, duration of diabetes and other chronic illnesses. Height, weight, waist circumference and hip circumference were measured for assessing the nutritional status and body mass index (BMI).
Patients sample collection and preparation
About 20 ml of fasting blood was collected in vacutainer tubes (purple tube-5 ml, yellow tubes-10 ml, and grey tube-5 ml) from each participant and stored on ice till processed. Plasma was separated from the specimen within 3 hours from collection. Serum samples were stored at -70°C and used within a month, if not analyzed on the same day. Fasting blood samples collected from all patients and control participants were used for measurement of plasma glucose, glycated hemoglobin, total antioxidant level, and total SOD enzyme activity.
Methods
Plasma glucose, glycated hemoglobin and lipid profile was measured by standard routine methods using ROCHE COBAS 6000 chemical auto analyzer by NHLS (National health laboratory services) of NMAH. The Total antioxidant level in serum was measured using a commercial kit from Sigma- Aldrich using ABTS method. Total SOD enzyme activity was measured using Cayman assay kits, USA (colorimetric method) BIO-TEK KC4 AUTOREADER was used for all the above analysis.
Statistical analysis
IBM statistical package for the Social Sciences (SPSS Inc, Chicago, IL, USA version 23) was used for statistical analysis. Data are expressed as mean ± SD (standard deviation) for normally distributed data and median (IQR) for data not normally distributed. The statistical significance of differences between the means of quantitative variable across groups (control, diabetics and diabetics with retinopathy) was evaluated by the one- way ANOVA (Analysis of Variance) test and Tukey HSD (Honest Significant Difference) as post-hoc test for variables with normal distribution. Non parametric Kruskal-Wallis test and Dunn’s post-hoc test with Bonferroni’s correction was done for variables not following normal distribution. Bivariate correlations were performed using Spearman rank correlation to analyze relationships between continuous variables. Multiple logistic regression analysis was used to calculate the Odds ratio for the odds of having diabetic retinopathy with different risk factors.
Results
Table 1 shows the information about the general profile of the participants. Among our study participants, females constituted about 62.1% where as 37.9% were males, 74.5% of participants were known hypertensive and belonged to diabetic groups. Healthy control (non-diabetic) group were all normotensive. Mean age did not show any significant difference among the groups (p=0.071). Waist/hip ratio was significantly different between groups (p<0.0005) and was higher in diabetic patients with and without retinopathy (median=0.93 and median=0.91) respectively compared to non-diabetic controls (median=0.87). There was no significant difference in waist/hip ratio among diabetic groups with and without retinopathy, p>0.05. Duration of diabetes is significantly higher in diabetic retinopathy (DR) group (median=10.0) as compared to diabetic group without retinopathy (median=5.0), p<0.0005 as seen in Table 2. Other measurements did not show any significant difference.
| Variables | GROUPS | |||
| Control Group n= 42 | Diabetic Group n=54 | DR Group n=44 | Total % N=140 | |
| Gender | ||||
| Female | n=27 (19.3%) | n=32 (22.9%) | n=28 (20.0%) | 0.621 |
| Male | n=15 (10.7%) | n=22 (15.7%) | n=16 (11.4%) | 0.379 |
| Hypertension | N/A | n=44 (44.9%) | n=29 (29.6%) | 74.5% ( N=98) |
Table 1: General Profile of all participants.
| Variables | GROUPS | |||
| Control Group n=42 | Diabetic Group n=54 | DR Group n=44 | P-value | |
| Age (Years) | 54.67 ± 6.76 | 56.50 ± 7.56 | 58.52 ± 8.66 | P=0.071 |
| Duration of Diabetes(Years) | N/A | 5 | 10 | P<0.0005*** |
| (8.25-2.0) | (15.0-5.0) | |||
| BMI (Kg/m2) | 29.7 | 31.3 | 31.7 | P=0.618 |
| (35.1-25.2) | (36.6-26.0) | (35.7-26.2) | ||
| Weight(Kg) | 77 | 80 | 80 | P=0.331 |
| (87.5-65.8) | (95.2-69.5) | (92.7-69.2) | ||
| Height(cm) | 160 | 162 | 160 | P=0.249 |
| (163.5-155.5) | (168.5-155) | (165-155) | ||
| Waist | 97.5 | 99 | 100 | P=0.135 |
| Circumference (cm) | (106-80.5) | (109-91) | (108.5-91) | |
| Hip | 107.5 | 106.5 | 105.5 | P=0.859 |
| Circumference (cm) | (122-93.8) | (118.2-99) | (116-99.5) | |
| Waist/hip Ratio | 0.87 | 0.91 | 0.93 | P<0.0005*** |
| (0.89-0.85) | (0.94-0.87) | (0.95-0.89) | ||
| Data is shown as mean ± SD (standard deviation)andmedian(IQR) (Interquartile range).Themean and median difference is significant at the *p<0.05, **p<0.005,***p<0.0005 level. DR-Diabetic Retinopathy | ||||
Table 2: Age, duration of diabetes and anthropometric data in groups.
Fasting plasma glucose and glycated hemoglobin levels were statistically significantly different between groups (p<0.0005 and p<0.0005) respectively. In diabetic patients with and without retinopathy, higher fasting plasma glucose and glycated hemoglobin were found compared to non-diabetic control group. The medians of FPG and glycated hemoglobin showed marginal increases in the DR group as compared to the diabetic group without retinopathy, but was not statistically significant (p>0.05). A statistically significant decrease was observed in the total antioxidant levels in the diabetic retinopathy group (median=0.34) as compared to the healthy control group (median=0.60, p<0.0005) and diabetic group without retinopathy (median=0.47, p=0.019). Diabetic group without retinopathy also showed significant decrease in TAO levels compared to healthy control group (p=0.048). SOD levels were statistically significantly increased in the diabetic retinopathy group (5.86 ± 2.03, p<0.0005) compared to healthy control group (3.70 ± 1.53) and diabetic group without retinopathy (4.19 ± 1.47, p<0.0005), but no other group differences were statistically significant (Table 3).
| Variables | GROUPS | |||
| Control Group n= 42 | Diabetic Group n= 42 | DR Group n= 42 | P-value | |
| FPG (mmol/l) | 4.9(5.2-4.6) | 8.1(10.5-6.3) | 9.6(13.1-6.5) | P<0.0005*** |
| HbA1c (%) | 6.0(6.1-5.8) | 8.1(10.2-6.8) | 9.0(11.1-8.0) | P<0.0005*** |
| Total antioxidant (mM) | 0.60(0.80-0.44) | 0.47(0.61-0.37) | 0.34(0.45-0.23) | P<0.0005*** |
| Serum SOD (U/ml) | 3.70 ± 1.53 | 4.19 ± 1.47 | 5.86 ± 2.03 | P<0.0005*** |
| Data is shown as mean ± SD (standard deviation) and median (IQR) (Interquartile range). The mean and median difference is significant at the *p<0.05, **p<0.005, ***p<0.0005 level. DR-Diabetic Retinopathy | ||||
Table 3: Clinical data in different groups.
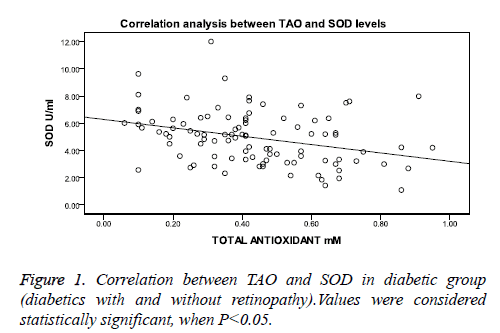
Correlation analysis between SOD and TAO levels showed significant negative correlation in combined diabetic group as seen in Figure 1, whereas in healthy control group the correlation was not significant (r=0.213, p= 0.186, n=40).
When correlation was performed in individual diabetic groups, the diabetic group without retinopathy showed a weak but significant negative correlation (r=-0.275, p=0.046) and the diabetic retinopathy group showed a negative correlation, but this was not statistically significant (r=-0.145, p=0.348). Multiple logistic regression analysis was carried out to check the risk factors for diabetic retinopathy. SOD [OR 1.91 (1.34-2.71), p<0.001] and duration of disease [OR 1.12 (1.01-1.24), p=0.027] were the only risk factors which were found to be significantly associated with the presence of retinopathy as observed in Table 4. Age, BMI, FPG, HbA1c, and TAO did not show any significance.
| variables | B | S.E | Wald | Sig. | Exp(B) | 95% CI for EXP(B) | |
| Lower | Upper | ||||||
| Age (years) | 0.038 | 0.037 | 1.028 | 0.311 | 1.039 | 0.965 | 1.117 |
| BMI (Kg/m2) | 0.02 | 0.042 | 0.22 | 0.639 | 1.02 | 0.94 | 1.106 |
| DD (years) | 0.114 | 0.052 | 4.864 | 0.027* | 1.121 | 1.013 | 1.24 |
| FPG (mmol/l) | 0.142 | 0.084 | 2.832 | 0.092 | 1.153 | 0.977 | 1.36 |
| HbA1c (%) | 0/147 | 0.142 | 1.079 | 0.299 | 1.159 | 0.877 | 1.531 |
| TAO (mM) | -1.243 | 1.37 | 0.823 | 0.354 | 0.289 | 0.02 | 4.231 |
| SOD (U/ml) | 0.646 | 0.179 | 12.967 | 0.000* | 1.908 | 1.342 | 2.711 |
| Constant | -9.09 | 3.445 | 6.963 | 0.008 | 0 | - | - |
| Abbreviations: BMI: Body Mass Index; DD: Duration of Diabetes; FPG:Fasting Plasma Glucose; HbA1c: glycosylated Haemoglobin; TAO: Total Antioxidant; SOD:Superoxide Dismutase. *p<0.05. | |||||||
Table 4: Independent markers associated with diabetic retinopathy among diabetic patients using multiple logistic regression analysis.
Discussion
In the present study, there was significant increase in the fasting plasma glucose and HbA1c in diabetic patients as compared to the control population. This is indicative of excessive glycosylation of hemoglobin due to increased plasma glucose or hyperglycemia as reported by other studies [23-27]. Chronic hyperglycemia results in oxidative stress by simulating reactive oxygen species (ROS) and reactive nitrogen species(RNS) production [28], which attacks lipids present in plasma, mitochondria and endoplasmic reticulum membranes and cause per oxidation [15]. In our study we observed a decreased total antioxidant status among diabetic patients and this decrease could be attributed to increased oxidative stress and excess utilization of antioxidants against oxidative stress to minimize the damage. EC-SOD is one of the three isoforms of SOD found in serum. It is a secretory glycoprotein with an affinity for heparin like substances and is the main enzymatic scavenger of superoxide in the extracellular spaces. 99% of the enzyme is found bound to heparin sulphate proteoglycans in vascular walls and to a lesser extent in the interstitium and 1% is in circulation. There is equilibrium between the plasma phase and endothelium phase [29]. The change in plasma SOD enzyme activity can be due to changes in expression of SOD or tissue binding of SOD [30].
Hyperglycemia in diabetes activates various biochemical pathways leading to increased production of superoxide and hydroxyl radical which could lead to decrease in SOD enzyme activity. But conflicting results have been reported in different studies. Most researches have reported a decrease in SOD enzyme activity in diabetic patients compared to healthy control group [29,31-35], whereas some did not report any changes [36] and few researchers reported high serum ECSOD enzyme activity in diabetic patients. Mizobuchi et al., Turk et al., Kimura et al., Soliman and Bandeira et al. all reported an increase in EC- SOD enzyme activity in diabetic group as compared to healthy control group. They also reported that this increase was seen more in patients with microangiopathy as compared to patients without complications, which is similar to the present study finding [11,37-40].
The reason for increase in the total SOD enzyme activity in the patients with retinopathy in this study could be due to, increased expression of the enzyme as a compensatory mechanism in response to increased oxidative stress. It may also be due to a reduced tissue binding of SOD as a result of glycation of SOD, which reduces the affinity of this enzyme to heparin without affecting its enzyme activity. In diabetes, the proportion of glycated SOD is higher than healthy control [41]. Also another reason could be decrease in tissue binding of SOD due to reduced heparin sulphate leading to an increase in plasma SOD levels. This reason is more evidence based as there have been reports that in diabetic nephropathy, heparan sulphate is reduced in glomerular basement membrane, proportional to degree of proteinuria and damage to glomerulus can also reduce the ability of membrane glycocalyx to bind SOD [11].
Also, there have been studies, where the authors have showed strong relationship between EC-SOD levels and severity of micro and macrovascular complications and have attributed this to a decrease in binding of this enzyme to the endothelium resulting in the vascular wall being more vulnerable to oxidative stress [11]. In the present study, we have also observed more increase in SOD enzyme activity in diabetic retinopathy patients compared to diabetics without retinopathy. Correlation analysis showed significant negative but weak correlation between TAO and SOD enzyme in the combined diabetic group (diabetic with and without retinopathy) as well as in the diabetic group without retinopathy. So it does indicate that with increase in oxidative stress, the increase in SOD enzyme could be due to depletion of antioxidants defenses and due to increase in superoxide production [40]. The lack of significant correlation in the diabetic retinopathy group could be due to the small sample size. Multiple logistic regression analysis did show the relationship between SOD and presence of diabetic retinopathy and was statistically significant along with duration of diabetes thus making it an important risk factor for diabetic retinopathy in our patients.
Conclusion
The results from the above study suggest that hyperglycemia in type 2 diabetes mellitus can cause oxidative stress and decreased total antioxidant levels as seen in our study. Also increase in total SOD enzyme activity in diabetic retinopathy patient’s points towards increase expression of this enzyme as a result of increased superoxide production and thus can be a probable oxidative stress biomarker. Although a study of the isoforms would give us a clear idea about whether it is the EC-SOD responsible for the increase in this study like reported by other studies.
Limitations
The study didn’t take into account the effect of medication (diabetes or hypertension medication) on SOD and TAO levels as the participants were not asked to stop any medications for the study. The effect of diet was not considered in the study and the participants were not subjected to any standard diet. The accuracy of information provided by the participants could have also been one of the drawbacks of our study. The small sample size in this study could affect the statistical analysis and results of the study.
Acknowledgements
The authors would like thank all the participants who made this study possible and Walter Sisulu University for funding this research project.
References
- Wold LE, Ceylan-lsik AF. Ren Oxidative stress and stress signaling: menace of diabetic Cardiomyopathy. Acta Pharmacologica Sinica 2005; 26: 908-917.
- Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res 2007; 1-12.
- Maiese K, Chong ZZ, ShangYC. Mechanistic Insights into Diabetes mellitus and Oxidative stress. Curr Med Chem 2007; 14: 1729-1738.
- Bertram MY, Jaswal AVS, Pillay Van Wyk V, Levitt NS, Hofman KJ. The non-fatal disease burden caused by type 2diabetesin South Africa, 2009. Glob Health Action 2013; 6: 19244.
- Read O, Cook C. Retinopathy in diabetic patients evaluated at a primary care clinic in Cape Town. JEMDSA 2007; 12: 56-64.
- Wu Y, Tang T, Chen B. Oxidative Stress: Implications for the Development of Diabetic Retinopathy and Antioxidant Therapeutic perspectives. Oxidative Med Cell Longevity 2014.
- Rema M, Pradeepa R. Diabetic retinopathy: An Indian perspective. Indian J Med Res 2007; 125: 297-310.
- Fowler MJ. Microvascular and Macrovascular Complications of Diabetes. Clin Diabetes 2008; 26: 77-82.
- Chang YC, Wu WC. Dyslipidemia and Diabetic Retinopathy. Rev Diabetes Studies 2013; 10: 121-132.
- Shin ES, Sorenson CM, Sheibani N. Diabetes and Retinal Vascular Dysfunction. J Ophthalmol Vision Res 2014; 9: 362-373.
- Kimura F, Hasegawa G, Obayashi H, Adachi T, Hara H, Ohta M, Fukui M, Kitagawa Y, Park H, Nakamura N, Nakano K, Yoshikawa T. Serum extracellular superoxide dismutase in patients with type 2 diabetes. Relationship to the development of micro and macrovascular complications. Diabetes Care 2003; 26: 1246-1250.
- Mates JM, Perez-Gomez C, Nunez De Castro I. Antioxidant Enzymes and Human Diseases. Clin Biochem 1999; 32: 595-603.
- Davies KJA. Oxidative stress, antioxidant defenses and damage removal, repair and replacement systems. IUBMB Life 2000; 50: 278-289.
- Atalay M, Laaksonen DE. Diabetes, oxidative stress and physical exercise. J Sport Sci Med 2002; 1: 1-14.
- Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of Diabetic Neuropathy. Endocrine Rev 2004; 25: 612-628.
- Fukai T, Ushio-Fukai M. Superoxide Dismutases: Role in Redox Signaling,Vascular Function,and Diseases. Antioxidants Redox Signal 2011; 15: 1583-1606.
- Tiwari BK, Pandey KB, Abidi AB, Rizvi SI. Markers of Oxidative Stress during Diabetes mellitus. J Biomarkers 2013.
- Crapo JD, Oury T, Rabouille C, Slot JW, Chang LY. Copper, zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci USA1992; 89: 10405-10409.
- Battin EE, Brumaghim JL. Antioxidant activity of Sulphur and Selenium: A Review of Reactive Oxygen Species Scavenging, Glutathione Peroxidase, and Metal-binding Antioxidant Mechanisms. Cell Biochem Biophys 2009; 55: 1-23.
- Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: A Review. Biochem Mol Toxicol 2003; 17: 24-38.
- Limón -Pacheco J, Gonsebatt ME. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutation Res 2009; 674: 137-147.
- Kohen R, Nyska A. Oxidation of Biological Systems: Oxidative Stress Phenomena, Antioxidants, Redox Reactions, and Methods of their Quantification. Toxicol Pathol 2002; 30: 620-650.
- Benrebai M, Abidli N, Nasr SM, Benlatreche C. Oxidative stress status in Type 2 diabetic patients in Eastern Algeria. World Appl Sci J 2008; 4: 714-719.
- Kharroubi AT, Darwish HM, Akkawi MA, Ashareef AA, Almasri ZA, Bader KA, Khammash UM. Total antioxidant status in type2 diabetic patients in Palestine. J Diabetes Res 2015.
- Pasupathi P, Bakthavathsalam G, Saravanan G, Latha R. Evaluation of oxidative stress and antioxidant status in patients with diabetes mellitus. J Appl Sci Res 2009; 5: 770-775.
- Merzouk S, Hichami A, Sari A, Madani S, Merzouk H. Impaired oxidant/antioxidant status and LDL-fatty acid composition are associated with increased susceptibility to peroxidation of LDL in diabetic patients. Gen Physiol Biophys 2004; 23: 387-399.
- Nourooz-Zadeh J, Rahimi A, Tajaddini-Sarmadi J, Tritschler H, Rosen P. Relationships between plasma measures of oxidative stress and metabolic control in NIDDM. Diabetologia 1997; 40: 647-653.
- Kuroki T, Isshiki K, King GL. Oxidative stress: the lead or supporting actor in the pathogenesis of diabetic complications. J Am Soc Nephrol 2003; 14: S216-220.
- Sayed AA, Aldebasi Y, Abd-allah SO, El-Gendy SM, Mohamed AS, Abd El-Fattah MS. Molecular and Biochemical study of superoxide dismutase gene polymorphisms in Egyptian patients with type 2 diabetes mellitus with and without retinopathy. Br J Med Med Res 2013; 3: 1258-1270.
- Adachi T, Inoue M, Hara H, Maehata E, Suzuki S. Relationship of plasma extracellular-superoxide dismutase level with insulin resistance in type 2 diabetic patients. J Endocrinol 2004; 181: 413-417.
- Hartnett ME, Stratton RD, Browne RW, Rosner BA, Lanham RJ. Serum markers of oxidative stress and severity of diabetic retinopathy. Diabetes Care 2000; 23: 234-240.
- Merzouk S, Hichami A, Madani S, Merzouk H, Yahia- Berrouiguet A, Prost J, Moutairou K, Chabane-Sari N, Khan NA. Antioxidant status and levels of different vitamins determined by high performance liquid chromatography in diabetic subjects with multiple complications. Gen Physiol Biophys 2003; 22: 15-27.
- Gupta M, Chari S. Proxidant and antioxidant status in patients of type II Diabetes Mellitus with IHD. Indian J Clin Biochem 2006; 21: 118-122.
- Goodarzi MT, Varmaziar L, Navidi AA, Parivar K. Study of oxidative stress in type 2 diabetic patients and its relationship with glycated hemoglobin. Saudi Med J 2008; 29: 503-506.
- Said NS, Hadhoud KM, Nada WM, El-Tarhouny SA. Superoxide dismutase, glutathione peroxidase and vitamin E in patients with diabetic retinopathy. Life Sci J 2013; 10: 1851-1856.
- Kesavulu MM, Giri R, Kameshwara Rao B, Apparao CH. Lipid peroxidation and Antioxidant enzyme levels in type 2 Diabetics with microvascular complications. Diabetes Metabol 2000; 26: 387-392.
- Mizobuchi N, Nakata H, Horimi T, Takahashi I. Serum superoxide dismutase (SOD) activity in diabetes mellitus. Rinsho Byori 1993; 41: 673-678.
- Turk HM, Sevinc A, Camci C, Cigli A, Buyukberber S. Plasma lipid peroxidation products and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Acta Diabetol 2002; 39: 117-122.
- Soliman GZ. Blood lipid peroxidation (superoxide dismutase, malondialdehyde, glutathione) levels in Egyptian type 2 diabetic patients. Singapore Med J 2008; 49: 129-136.
- Bandeira SM, Guedes GS, Fonseca LJS, Pires AS, Gelain DP, Moreira JCF, Rabelo LA, Vasconcelos SML, Goulart MOF. Characterization of blood Oxidative stress in type 2 diabetes mellitus patients: increase in lipid peroxidation and SOD activity. Oxidative Med Cell Longevity 2012.
- Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab 2000; 26: 163-176.